Dominant-negative effects of COL7A1 mutations can be rescued by controlled overexpression of normal collagen VII
- PMID: 19726672
- PMCID: PMC2781580
- DOI: 10.1074/jbc.M109.045294
Dominant-negative effects of COL7A1 mutations can be rescued by controlled overexpression of normal collagen VII
Abstract
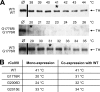
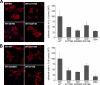
Dominant-negative interference by glycine substitution mutations in the COL7A1 gene causes dominant dystrophic epidermolysis bullosa (DDEB), a skin fragility disorder with mechanically induced blistering. Although qualitative and quantitative alterations of the COL7A1 gene product, collagen VII, underlie DDEB, the lack of direct correlation between mutations and the clinical phenotype has rendered DDEB less amenable to therapeutic targeting. To delineate the molecular mechanisms of DDEB, we used recombinant expression of wild-type (WT) and mutant collagen VII, which contained a naturally occurring COL7A1 mutation, G1776R, G2006D, or G2015E, for characterization of the triple helical molecules. The mutants were co-expressed with WT in equal amounts and could form heterotrimeric hybrid triple helices, as demonstrated by affinity purification and mass spectrometry. The thermal stability of the mutant molecules was strongly decreased, as evident in their sensitivity to trypsin digestion. The helix-to-coil transition, T(m), of the mutant molecules was 31-34 degrees C, and of WT collagen VII 41 degrees C. Co-expression of WT with G1776R- or G2006D-collagen VII resulted in partial intracellular retention of the collagen, and mutant collagen VII had reduced ability to support cell adhesion. Intriguingly, controlled overexpression of WT collagen VII gradually improved the thermal stability of the collective of collagen VII molecules. Co-expression in a ratio of 90% WT:10% mutant increased the T(m) to 41 degrees C for G1776R-collagen VII and to 39 degrees C for G2006D- and G2015E-collagen VII. Therefore, increasing the expression of WT collagen VII in the skin of patients with DDEB can be considered a valid therapeutic approach.
Figures





References
-
- Kern J. S., Has C. (2008) Expert. Rev. Dermatol. 3, 721–733
-
- Fine J. D., Eady R. A., Bauer E. A., Bauer J. W., Bruckner-Tuderman L., Heagerty A., Hintner H., Hovnanian A., Jonkman M. F., Leigh I., McGrath J. A., Mellerio J. E., Murrell D. F., Shimizu H., Uitto J., Vahlquist A., Woodley D., Zambruno G. (2008) J. Am. Acad. Dermatol. 58, 931–950 - PubMed
-
- Rattenholl A., Pappano W. N., Koch M., Keene D. R., Kadler K. E., Sasaki T., Timpl R., Burgeson R. E., Greenspan D. S., Bruckner-Tuderman L. (2002) J. Biol. Chem. 277, 26372–26378 - PubMed
-
- Burgeson R. E. (1993) J. Invest. Dermatol. 101, 252–255 - PubMed
-
- Kadler K. E., Baldock C., Bella J., Boot-Handford R. P. (2007) J. Cell Sci. 120, 1955–1958 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

