Expression of glycine-activated diheteromeric NR1/NR3 receptors in human embryonic kidney 293 cells Is NR1 splice variant-dependent
- PMID: 19726695
- PMCID: PMC2784711
- DOI: 10.1124/jpet.109.158493
Expression of glycine-activated diheteromeric NR1/NR3 receptors in human embryonic kidney 293 cells Is NR1 splice variant-dependent
Abstract
In oocytes, glycine activates receptors formed by diheteromeric combinations of N-methyl-d-aspartate (NMDA) NR1 and NR3 subunits. In contrast, functional receptors in mammalian cells require the simultaneous expression of NR1 and both NR3A and NR3B subunits. In vivo, NR3A and NR3B subunits show differential expression patterns and thus may not naturally form triheteromeric receptors. In this study, we examined whether NR1 splice variants play a role in allowing assembly of functional diheteromeric receptors in mammalian cells. Little current was found in human embryonic kidney 293 cells coexpressing either NR3A or NR3B and the NR1-1a splice variant. However, robust glycine-activated currents were generated in cells transfected with NR3(A or B) and either NR1-2a, NR1-3a, or NR1-4a, and current density was correlated with NR1 C-terminal length. Truncation of the NR1-1a C terminus modestly enhanced NR1-1a/NR3A currents, whereas only small increases were observed with mutations of C-terminal residues that control trafficking or phosphorylation. In contrast, large currents were observed when an extracellular phenylalanine in NR1-1a that influences glycine access was mutated to alanine. A separate mutation in NR1-1a that disrupts glycine binding did not generate responses in NR1-1a/NR3A receptors alone, but it produced a greater than 30-fold potentiation of currents during coapplication of glycine and the glycine antagonist 7-chlorokynurenic acid. Finally, transfection of cells with the NR1-4a subunit along with NR2 and NR3 subunits resulted in the expression of both NR1/NR3 receptors and conventional NMDA receptor currents. These results indicate a prominent role for NR1 splice variants in the functional expression of NR1/NR3 receptors in mammalian cells.
Figures

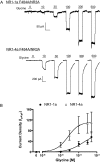
 ) and C-terminal domain (C1 cassette, ▥; C2 cassette, ▤; and C2′ cassette, ▨). Gray bars represent transmembrane domains. B, representative traces from NR1-1a/NR3A, NR1-2a/NR3A, NR1-3a/NR3A, and NR1-4a/NR3A recombinant receptors during exposure to 100 μM glycine. The horizontal bar above the traces indicates a 6-s agonist application. Scale bar, 5 s. C, current density relationship for glycine activation of NR1/NR3 receptors containing NR1-1a, NR1-2a, NR1-3a, and NR1-4a splice variants. NR1-1a/NR3A relationship is shown as a dash line for emphasis. The data represent peak current measurements normalized to whole-cell capacitance and expressed as mean ± S.E.M. (n = 9–14 cells per receptor combination). D, concentration-response relationship for glycine activation of NR1/NR3A receptors containing NR1-1a, NR1-2a, NR1-3a, and NR1-4a splice variants. The NR1-1a/NR3A relationship is shown as a dotted line for clarity. Data represent the peak current values expressed as a percentage (mean ± S.E.M.) of that obtained at the maximal glycine concentration (500 μM) tested (n = 10–15 determinations per glycine concentration).
) and C-terminal domain (C1 cassette, ▥; C2 cassette, ▤; and C2′ cassette, ▨). Gray bars represent transmembrane domains. B, representative traces from NR1-1a/NR3A, NR1-2a/NR3A, NR1-3a/NR3A, and NR1-4a/NR3A recombinant receptors during exposure to 100 μM glycine. The horizontal bar above the traces indicates a 6-s agonist application. Scale bar, 5 s. C, current density relationship for glycine activation of NR1/NR3 receptors containing NR1-1a, NR1-2a, NR1-3a, and NR1-4a splice variants. NR1-1a/NR3A relationship is shown as a dash line for emphasis. The data represent peak current measurements normalized to whole-cell capacitance and expressed as mean ± S.E.M. (n = 9–14 cells per receptor combination). D, concentration-response relationship for glycine activation of NR1/NR3A receptors containing NR1-1a, NR1-2a, NR1-3a, and NR1-4a splice variants. The NR1-1a/NR3A relationship is shown as a dotted line for clarity. Data represent the peak current values expressed as a percentage (mean ± S.E.M.) of that obtained at the maximal glycine concentration (500 μM) tested (n = 10–15 determinations per glycine concentration).



Similar articles
-
Pharmacological characterization of glycine-activated currents in HEK 293 cells expressing N-methyl-D-aspartate NR1 and NR3 subunits.J Pharmacol Exp Ther. 2007 Aug;322(2):739-48. doi: 10.1124/jpet.107.123836. Epub 2007 May 14. J Pharmacol Exp Ther. 2007. PMID: 17502428
-
Formation of NR1/NR2 and NR1/NR3 heterodimers constitutes the initial step in N-methyl-D-aspartate receptor assembly.J Biol Chem. 2008 Jan 4;283(1):37-46. doi: 10.1074/jbc.M703539200. Epub 2007 Oct 24. J Biol Chem. 2008. PMID: 17959602
-
Effects of NR1 splicing on NR1/NR3B-type excitatory glycine receptors.BMC Neurosci. 2009 Apr 6;10:32. doi: 10.1186/1471-2202-10-32. BMC Neurosci. 2009. PMID: 19348678 Free PMC article.
-
New insights into the not-so-new NR3 subunits of N-methyl-D-aspartate receptor: localization, structure, and function.Mol Pharmacol. 2010 Jul;78(1):1-11. doi: 10.1124/mol.110.064006. Epub 2010 Apr 2. Mol Pharmacol. 2010. PMID: 20363861 Review.
-
Influence of the NR3A subunit on NMDA receptor functions.Prog Neurobiol. 2010 May;91(1):23-37. doi: 10.1016/j.pneurobio.2010.01.004. Epub 2010 Jan 25. Prog Neurobiol. 2010. PMID: 20097255 Free PMC article. Review.
Cited by
-
Site of Action of Brain Neurosteroid Pregnenolone Sulfate at the N-Methyl-D-Aspartate Receptor.J Neurosci. 2020 Jul 29;40(31):5922-5936. doi: 10.1523/JNEUROSCI.3010-19.2020. Epub 2020 Jul 1. J Neurosci. 2020. PMID: 32611707 Free PMC article.
-
Moderate prenatal alcohol exposure reduces plasticity and alters NMDA receptor subunit composition in the dentate gyrus.J Neurosci. 2013 Jan 16;33(3):1062-7. doi: 10.1523/JNEUROSCI.1217-12.2013. J Neurosci. 2013. PMID: 23325244 Free PMC article.
-
Block of NMDA receptor channels by endogenous neurosteroids: implications for the agonist induced conformational states of the channel vestibule.Sci Rep. 2015 Jun 18;5:10935. doi: 10.1038/srep10935. Sci Rep. 2015. PMID: 26086919 Free PMC article.
-
Kinetic models for activation and modulation of NMDA receptor subtypes.Curr Opin Physiol. 2018 Apr;2:114-122. doi: 10.1016/j.cophys.2018.02.002. Epub 2018 Feb 22. Curr Opin Physiol. 2018. PMID: 29978141 Free PMC article.
-
Unmasking GluN1/GluN3A excitatory glycine NMDA receptors.Nat Commun. 2018 Nov 13;9(1):4769. doi: 10.1038/s41467-018-07236-4. Nat Commun. 2018. PMID: 30425244 Free PMC article.
References
-
- Awobuluyi M, Yang J, Ye Y, Chatterton JE, Godzik A, Lipton SA, Zhang D. (2007) Subunit-specific roles of glycine-binding domains in activation of NR1/NR3 N-methyl-d-aspartate receptors. Mol Pharmacol 71:112–122 - PubMed
-
- Chatterton JE, Awobuluyi M, Premkumar LS, Takahashi H, Talantova M, Shin Y, Cui J, Tu S, Sevarino KA, Nakanishi N, et al. ( 2002) Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature 415:793–798 - PubMed
-
- Cull-Candy S, Brickley S, Farrant M. (2001) NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol 11:327–335 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

