Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery
- PMID: 19726714
- PMCID: PMC2777767
- DOI: 10.1152/ajpregu.00343.2009
Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery
Abstract
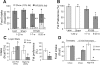
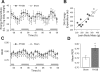
Gastric bypass surgery efficiently and lastingly reduces excess body weight and reverses type 2 diabetes in obese patients. Although increased energy expenditure may also play a role, decreased energy intake is thought to be the main reason for weight loss, but the mechanisms involved are poorly understood. Therefore, the aim of this study was to characterize the changes in ingestive behavior in a rat model of Roux-en-Y gastric bypass surgery (RYGB). Obese (24% body fat compared with 18% in chow-fed controls), male Sprague-Dawley rats maintained for 15 wk before and 4 mo after RYGB or sham-surgery on a two-choice low-fat/high-fat diet, were subjected to a series of tests assessing energy intake, meal patterning, and food choice. Although sham-operated rats gained an additional 100 g body wt during the postoperative period, RYGB rats lost approximately 100 g. Intake of a nutritionally complete and palatable liquid diet (Ensure) was significantly reduced by approximately 50% during the first 2 wk after RYGB compared with sham surgery. Decreased intake was the result of greatly reduced meal size with only partial compensation by meal frequency, and a corresponding increase in the satiety ratio. Similar results were obtained with solid food (regular or high-fat chow) 6 wk after surgery. In 12- to 24-h two-choice liquid or solid diet paradigms with nutritionally complete low- and high-fat diets, RYGB rats preferred the low-fat choice (solid) or showed decreased acceptance for the high-fat choice (liquid), whereas sham-operated rats preferred the high-fat choices. A separate group of rats offered chow only before surgery completely avoided the solid high-fat diet in a choice paradigm. The results confirm anecdotal reports of "nibbling" behavior and fat avoidance in RYGB patients and provide a basis for more mechanistic studies in this rat model.
Figures
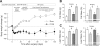
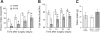
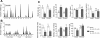
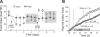
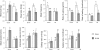
Similar articles
-
Longitudinal assessment of food intake, fecal energy loss, and energy expenditure after Roux-en-Y gastric bypass surgery in high-fat-fed obese rats.Obes Surg. 2013 Apr;23(4):531-40. doi: 10.1007/s11695-012-0846-2. Obes Surg. 2013. PMID: 23269513 Free PMC article.
-
Body Composition, Food Intake, and Energy Expenditure in a Murine Model of Roux-en-Y Gastric Bypass Surgery.Obes Surg. 2016 Sep;26(9):2173-2182. doi: 10.1007/s11695-016-2062-y. Obes Surg. 2016. PMID: 26781597 Free PMC article.
-
Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery.Endocrinology. 2010 Apr;151(4):1588-97. doi: 10.1210/en.2009-1332. Epub 2010 Feb 23. Endocrinology. 2010. PMID: 20179262 Free PMC article.
-
Feeding behavior and body weight development: lessons from rats subjected to gastric bypass surgery or high-fat diet.J Physiol Pharmacol. 2009 Dec;60 Suppl 7:25-31. J Physiol Pharmacol. 2009. PMID: 20388943 Review.
-
Physiological mechanisms behind Roux-en-Y gastric bypass surgery.Dig Surg. 2014;31(1):13-24. doi: 10.1159/000354319. Epub 2014 May 8. Dig Surg. 2014. PMID: 24819493 Review.
Cited by
-
Appetite and body weight regulation after bariatric surgery.Obes Rev. 2015 Feb;16 Suppl 1(Suppl 1):77-90. doi: 10.1111/obr.12258. Obes Rev. 2015. PMID: 25614206 Free PMC article. Review.
-
Surgical models of Roux-en-Y gastric bypass surgery and sleeve gastrectomy in rats and mice.Nat Protoc. 2015 Mar;10(3):495-507. doi: 10.1038/nprot.2015.027. Epub 2015 Feb 26. Nat Protoc. 2015. PMID: 25719268 Free PMC article.
-
Neural control of energy balance: translating circuits to therapies.Cell. 2015 Mar 26;161(1):133-145. doi: 10.1016/j.cell.2015.02.023. Cell. 2015. PMID: 25815991 Free PMC article. Review.
-
Early Postoperative Exposure to High-Fat Diet Does Not Increase Long-Term Weight Loss or Fat Avoidance After Roux-en-Y Gastric Bypass in Rats.Front Nutr. 2022 Apr 13;9:834854. doi: 10.3389/fnut.2022.834854. eCollection 2022. Front Nutr. 2022. PMID: 35495960 Free PMC article.
-
A Preventive Prebiotic Supplementation Improves the Sweet Taste Perception in Diet-Induced Obese Mice.Nutrients. 2019 Mar 5;11(3):549. doi: 10.3390/nu11030549. Nutrients. 2019. PMID: 30841548 Free PMC article.
References
-
- Berthoud HR, Carlson NR, Powley TL. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol Regul Integr Comp Physiol 260: R200–R207, 1991 - PubMed
-
- Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 85: 1–17, 2000 - PubMed
-
- Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG. Biliopancreatic diversion in rats is associated with intestinal hypertrophy and with increased GLP-1, GLP-2 and PYY levels. Obes Surg 17: 1193–1198, 2007 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

