Pathologic findings in retinal pigment epithelial cell implantation for Parkinson disease
- PMID: 19726750
- PMCID: PMC2764397
- DOI: 10.1212/WNL.0b013e3181bbff1c
Pathologic findings in retinal pigment epithelial cell implantation for Parkinson disease
Abstract
Background: Attempts at cell-based dopamine replacement therapy in Parkinson disease (PD) have included surgical implantation of adrenal medullary, fetal mesencephalic, and cultured human mesencephalic tissue grafts. Trials involving putamenal implantation of human retinal pigment epithelial (RPE) cells in PD have also been performed. Neuropathologic findings in humans undergoing RPE cell implantation have not heretofore been reported. We describe the brain autopsy findings from a subject enrolled in a clinical trial of RPE cells in gelatin microcarriers for treatment of PD, and suggest factors which may have impacted cell survival.
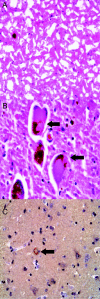
Methods: A 68-year-old man underwent bilateral surgical implantation of 325,000 RPE cells in gelatin microcarriers (Spheramine) but died 6 months after surgery. The left cerebral hemisphere was examined. Routine postmortem formalin fixation was performed and standard, as well as immunohistochemical methods used to highlight senile plaque and Lewy body pathologic changes, iron deposition, cellular inflammation, and reactive astrocytosis in implant regions. Manual cell counts were done of RPE cells.
Results: Hematoxylin-eosin and alpha-synuclein immunostains confirmed the diagnosis of PD. Needle tracts with matrix material and RPE cells were observed in the context of local inflammatory and astrocytic reactive change. A total of 118 cells were counted (estimated 0.036% survival).
Conclusions: Retinal pigment epithelial cells are seen in human brain 6 months postimplantation, but overall survival of implanted cells appeared poor.
Figures


Comment in
-
Autopsy investigation of neurodegenerative disease transplant patients: hindsight is 20/20.Neurology. 2009 Oct 6;73(14):1086-7. doi: 10.1212/WNL.0b013e3181bc6758. Epub 2009 Sep 2. Neurology. 2009. PMID: 19726748 No abstract available.
Similar articles
-
Intrastriatal implantation of human retinal pigment epithelial cells attached to microcarriers in advanced Parkinson disease.Arch Neurol. 2005 Dec;62(12):1833-7. doi: 10.1001/archneur.62.12.1833. Arch Neurol. 2005. PMID: 16344341 Clinical Trial.
-
Stereotaxic intrastriatal implantation of human retinal pigment epithelial (hRPE) cells attached to gelatin microcarriers: a potential new cell therapy for Parkinson's disease.J Neural Transm Suppl. 2003;(65):215-27. doi: 10.1007/978-3-7091-0643-3_14. J Neural Transm Suppl. 2003. PMID: 12946059 Review.
-
Spheramine for treatment of Parkinson's disease.Neurotherapeutics. 2008 Apr;5(2):252-9. doi: 10.1016/j.nurt.2008.02.006. Neurotherapeutics. 2008. PMID: 18394567 Free PMC article. Review.
-
Characterization and survival of long-term implants of human retinal pigment epithelial cells attached to gelatin microcarriers in a model of Parkinson disease.J Neuropathol Exp Neurol. 2007 Jul;66(7):585-96. doi: 10.1097/nen.0b013e318093e53a. J Neuropathol Exp Neurol. 2007. PMID: 17620984
-
Intraocular implantation of DNA-transfected retinal pigment epithelium cells: a new approach for analyzing molecular functions in the newt retinal regeneration.Neurosci Lett. 2004 Sep 23;368(2):171-5. doi: 10.1016/j.neulet.2004.07.009. Neurosci Lett. 2004. PMID: 15351443
Cited by
-
Is the Immunological Response a Bottleneck for Cell Therapy in Neurodegenerative Diseases?Front Cell Neurosci. 2020 Aug 11;14:250. doi: 10.3389/fncel.2020.00250. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32848630 Free PMC article. Review.
-
Cell based therapies in Parkinson's Disease.Ann Neurosci. 2011 Apr;18(2):76-83. doi: 10.5214/ans.0972.7531.1118209. Ann Neurosci. 2011. PMID: 25205926 Free PMC article. Review.
-
Neonatal human retinal pigment epithelial cells secrete limited trophic factors in vitro and in vivo following striatal implantation in parkinsonian rats.J Neural Transm (Vienna). 2016 Mar;123(3):167-77. doi: 10.1007/s00702-015-1480-7. Epub 2015 Nov 6. J Neural Transm (Vienna). 2016. PMID: 26546037
-
The future of stem cell therapies for Parkinson disease.Nat Rev Neurosci. 2020 Feb;21(2):103-115. doi: 10.1038/s41583-019-0257-7. Epub 2020 Jan 6. Nat Rev Neurosci. 2020. PMID: 31907406 Review.
-
Cell-therapy for Parkinson's disease: a systematic review and meta-analysis.J Transl Med. 2023 Sep 7;21(1):601. doi: 10.1186/s12967-023-04484-x. J Transl Med. 2023. PMID: 37679754 Free PMC article.
References
-
- Freed CR, Greene PE, Breeze RE, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med 2001;344:710–719. - PubMed
-
- Olanow CW, Koller W, Goetz CG, et al. Autologous transplantation of adrenal medulla in Parkinson's disease: 18-month results. Arch Neurol 1990;47:1286–1289. - PubMed
-
- Olanow CW, Kordower JH, Freeman TB. Fetal nigral transplantation as a therapy for Parkinson's disease. Trends Neurosci 1996;19:102–109. - PubMed
-
- Hauser RA, Freeman TB, Snow BJ, et al. Long-term evaluation of bilateral fetal nigral transplantation in Parkinson disease. Arch Neurol 1999;56:179–187. - PubMed
-
- Olanow CW, Goetz CG, Kordower JH, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol 2003;54: 403–414. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
