Safety of carbamazepine extended-release capsules used in combination with other psychotropic medications for the treatment of bipolar I disorder
- PMID: 19727252
- PMCID: PMC2686643
Safety of carbamazepine extended-release capsules used in combination with other psychotropic medications for the treatment of bipolar I disorder
Abstract
Objective: To evaluate the safety and efficacy of carbamazepine extended-release capsules (CBZ-ERC) in combination with other psychotropic medications for the treatment of bipolar I disorder.
Design: In this Phase IIIb, open-label, eight-week, observational, polypharmacy study, adult subjects were started on CBZ-ERC 200mg and titrated over four weeks to optimal dose (1600mg/d maximum). Concomitant lithium and atypical antipsychotics (olanzapine, risperidone, quetiapine, aripiprazole) were permitted. Safety assessments included adverse events, laboratory parameters, physical examination, medication history, vital signs, and electrocardiogram. Efficacy measures included the Young Mania Rating Scale (YMRS), Hamilton Rating Scale for Depression (HAM-D), Montgomery-Asberg Depression Rating Scale (MADRS), and Clinical Global Impressions Scale-Bipolar Version (CGI-BP). All data were summarized using descriptive statistics.
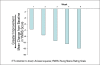
Results: Overall, 45 (84.9%) subjects reported treatment-emergent adverse events (TEAEs); most were mild or moderate in severity. The most commonly reported TEAEs were somnolence (n=14, 26.4%), sedation (n=12, 22.6%), dizziness (n=11, 20.8%), headache (n=9, 17.0%), and nausea (n=7, 13.2%). There were no clinically significant changes in vital signs, including weight. Mean changes in laboratory parameters were small, with values that were within the normal range for the majority of subjects. Few changes relative to screening for other safety parameters occurred. Mean total YMRS score decreased from baseline at each study visit. HAM-D and MADRS scores decreased from baseline at Weeks 4 and 8, and all three CGI-BP components (overall bipolar disorder, mania, and depression) improved during the study.
Conclusion: CBZ-ERC appears to be safe and effective for use in combination with atypical antipsychotics and lithium for treatment of bipolar I disorder.
Keywords: bipolar disorder; carbamazepine; extended release.
Figures
References
-
- Poolsup N, Li Wan PA, de Oliveira IR.Systematic overview of lithium treatment in acute mania. J Clin Pharm Ther. 200025139–156 - PubMed
-
- Bowden CL, Brugger AM, Swann AC, et al. Efficacy of divalproex vs lithium and placebo in the treatment of mania. The Depakote Mania Study Group. JAMA. 1994271918–924 - PubMed
-
- Pope HG, Jr, McElroy SL, Keck PE, Jr, et al. Valproate in the treatment of acute mania. A placebo-controlled study. Arch Gen Psychiatry. 19914862–68 - PubMed
-
- Weisler RH, Kalali AH, Ketter TA.A multicenter, randomized, double-blind, placebo-controlled trial of extended-release carbamazepine capsules as monotherapy for bipolar disorder patients with manic or mixed episodes. J Clin Psychiatry. 200465478–484 - PubMed
-
- Weisler RH, Keck PE, Jr, Swann AC, et al. Extended-release carbamazepine capsules as monotherapy for acute mania in bipolar disorder: a multicenter, randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 200566323–330 - PubMed
LinkOut - more resources
Full Text Sources