Immunobiological outcomes of repeated chlamydial infection from two models of within-host population dynamics
- PMID: 19727394
- PMCID: PMC2731222
- DOI: 10.1371/journal.pone.0006886
Immunobiological outcomes of repeated chlamydial infection from two models of within-host population dynamics
Abstract
Background: Chlamydia trachomatis is a common human pathogen that mediates disease processes capable of inflicting serious complications on reproduction. Aggressive inflammatory immune responses are thought to not only direct a person's level of immunity but also the potential for immunopathology. With human immunobiology being debated as a cause of prevailing epidemiological trends, we examined some fundamental issues regarding susceptibility to multiple chlamydial infections that could have implications for infection spread. We argue that, compared to less-frequent exposure, frequent exposure to chlamydia may well produce unique immunobiological characteristics that likely to have important clinical and epidemiological implications.
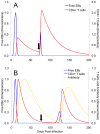
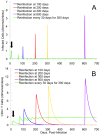
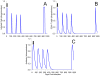
Methods and results: As a novel tool for studying chlamydia, we applied principles of modeling within-host pathogen dynamics to enable an understanding of some fundamental characteristics of an individual's immunobiology during multiple chlamydial infections. While the models were able to reproduce shorter-term infection kinetics of primary and secondary infections previously observed in animal models, it was also observed that longer periods between initial and second infection may increase an individual's chlamydial load and lengthen their duration of infectiousness. The cessation of short-term repeated exposure did not allow for the formation of long-lasting immunity. However, frequent re-exposure non-intuitively linked the formation of protective immunity, persistent infection, and the potential for immunopathology.
Conclusions: Overall, these results provide interesting insights that should be verified with continued study. Nevertheless, these results appear to raise challenges for current evidence of the development of long-lasting immunity against chlamydia, and suggest the existence of a previously unidentified mechanism for the formation of persistent infection. The obvious next goal is to investigate the qualitative impact of these results on the spread of chlamydia.
Conflict of interest statement
Figures

 kUE. Basic and extended models are labeled by curved braces. Dashed arrows between state variables indicate interactions between them.
kUE. Basic and extended models are labeled by curved braces. Dashed arrows between state variables indicate interactions between them.

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

