The flexible pocketome engine for structural chemogenomics
- PMID: 19727619
- PMCID: PMC2975493
- DOI: 10.1007/978-1-60761-274-2_11
The flexible pocketome engine for structural chemogenomics
Abstract
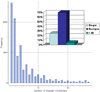
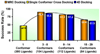
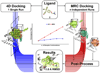
Biological metabolites, substrates, cofactors, chemical probes, and drugs bind to flexible pockets in multiple biological macromolecules to exert their biological effect. The rapid growth of the structural databases and sequence data, including SNPs and disease-related genome modifications, complemented by the new cutting-edge 3D docking, scoring, and profiling methods has created a unique opportunity to develop a comprehensive structural map of interactions between any small molecule and biopolymers. Here we demonstrate that a comprehensive structural genomics engine can be built using multiple pocket conformations, experimentally determined or generated with a variety of modeling methods, and new efficient ensemble docking algorithms. In contrast to traditional ligand-activity-based engines trained on known chemical structures and their activities, the structural pocketome and docking engine will allow prediction of poses and activities for new, previously unknown, protein binding sites, and new, previously uncharacterized, chemical scaffolds. This de novo structure-based activity prediction engine may dramatically accelerate the discovery of potent and specific therapeutics with reduced side effects.
Figures






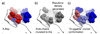


References
-
- Hendlich M, Bergner A, Gunther J, Klebe G. Relibase: design and development of a database for comprehensive analysis of protein-ligand interactions. J Mol Biol. 2003;326:607–620. - PubMed
-
- Kuhn D, Weskamp N, Hullermeier E, Klebe G. Functional classification of protein kinase binding sites using Cavbase. ChemMedChem. 2007;2:1432–1447. - PubMed
-
- An J, Totrov M, Abagyan R. Pocketome via Comprehensive Identification and Classification of Ligand Binding Envelopes. Mol Cell Proteomics. 2005;4:752–761. - PubMed
-
- Cavasotto CN, Orry AJW, Murgolo NJ, Czarniecki MF, Kocsi SA, Hawes BE;x, x, Neill KA, Hine H, Burton MS, Voigt JH, Abagyan RA, Bayne ML, Monsma FJ. Discovery of Novel Chemotypes to a G-Protein-Coupled Receptor through Ligand-Steered Homology Modeling and Structure-Based Virtual Screening. J. Med. Chem. 2008;51:581–588. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

