High-content drug screening with engineered musculoskeletal tissues
- PMID: 19728786
- PMCID: PMC2865990
- DOI: 10.1089/ten.TEB.2009.0445
High-content drug screening with engineered musculoskeletal tissues
Abstract
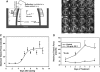
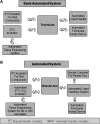
Tissue engineering for in vitro drug-screening applications based on tissue function is an active area of translational research. Compared to targeted high-throughput drug-screening methods that rapidly analyze hundreds of thousands of compounds affecting a single biochemical reaction or gene expression, high-content screening (HCS) with engineered tissues is more complex and based on the cumulative positive and negative effects of a compound on the multiple pathways altering tissue function. It may therefore serve as better predictor of in vivo activity and serve as a bridge between high-throughput drug screening and in vivo animal studies. In the case of the musculoskeletal system, tissue function includes determining improvements in the mechanical properties of bone, tendon, cartilage, and, for skeletal muscle, contractile properties such as rate of contraction/relaxation, force generation, fatigability, and recovery from fatigue. HCS of compound banks with engineered tissues requires miniature musculoskeletal organs as well as automated functional testing. The resulting technologies should be rapid, cost effective, and reduce the number of small animals required for follow-on in vivo studies. Identification of compounds that improve the repair/regeneration of damaged tissues in vivo would have extensive clinical applications for treating musculoskeletal disorders.
Figures



References
-
- Vandenburgh H.H. Swasdison S. Karlisch P. Computer aided mechanogenesis of skeletal muscle organs from single cells in vitro. FASEB J. 1991;5:2860. - PubMed
-
- Langer R. Vacanti J.P. Tissue engineering. Science. 1993;260:920. - PubMed
-
- Griffith L.G. Naughton G. Tissue engineering—current challenges and expanding opportunities. Science. 2002;295:1009. - PubMed
-
- Terstappen G.C. Schlupen C. Raggiaschi R. Gaviraghi G. Target deconvolution strategies in drug discovery. Nat Rev Drug Discov. 2007;6:891. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

