Evaluation of trial outcomes in acute kidney injury by creatinine modeling
- PMID: 19729431
- PMCID: PMC2774954
- DOI: 10.2215/CJN.00820209
Evaluation of trial outcomes in acute kidney injury by creatinine modeling
Abstract
Background and objectives: Clinical trials of acute kidney injury (AKI) use changes in creatinine as outcome metrics. This study investigated how outcome metrics and baseline creatinine affect trial outcome.
Design, setting, participants, & measurements: A one-compartment pharmacokinetic model of creatinine change resulting from a decrease in GFR was applied to a population of 10,000 simulated virtual inpatients. Treatment was simulated as an amelioration of GFR decrease by a specified percentage, the treatment efficacy, in 50%. Three categorical and two continuous outcome metrics were calculated and compared. Outcomes were compared for measured and estimated baseline creatinine levels that were back-calculated assuming a GFR of 100 or 75 ml/min.
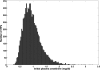
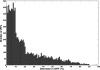
Results: The continuous metrics, the average value of creatinine and the average value of creatinine relative to baseline decreased approximately linearly with increase in treatment efficacy. The categorical metrics displayed a sigmoidal decrease and erroneously suggested perfect treatment when GFR decrease was ameliorated by only 60 to 80%. Using an estimate of baseline creatinine increased the number of patients who were classified as having AKI.
Conclusions: When used to determine clinical trial outcome, continuous metrics correctly detected the extent of intervention. At low treatment efficacy, categorical metrics underestimated and at high treatment efficacy overestimated the effect of treatment. These effects were exaggerated when the population contained a high proportion of patients with more severe AKI. An estimated baseline creatinine level will overestimate AKI prevalence compared with a measured baseline value. Clinical trials of AKI should use a continuous outcome metric and a measured baseline and report baseline median and interquartile range.
Figures







References
-
- Westhuyzen J, Endre ZH, Reece G, Reith DM, Saltissi D, Morgan TJ: Measurement of tubular enzymuria facilitates early detection of acute renal impairment in the intensive care unit. Nephrol Dial Transplant 18: 543– 551, 2003 - PubMed
-
- Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P: Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365: 1231– 1238, 2005 - PubMed
-
- Macedo E, Abdulkader R, Castro I, Sobrinho AC, Yu L, Vieira JM: Lack of protection of N-acetylcysteine (NAC) in acute renal failure related to elective aortic aneurysm repair-a randomized controlled trial. Nephrol Dial Transplant 21: 1863– 1869, 2006 - PubMed
-
- Marathias KP, Vassili M, Robola A, Alivizatos PA, Palatianos GM, Geroulanos S, Vlahakos DV: Preoperative intravenous hydration confers renoprotection in patients with chronic kidney disease undergoing cardiac surgery. Artif Organs 30: 615– 621, 2006 - PubMed
-
- Haase M, Haase-Fielitz A, Bagshaw SM, Reade MC, Morgera S, Seevenayagam S, Matalanis G, Buxton B, Doolan L, Bellomo R: Phase II, randomized, controlled trial of high-dose N-acetylcysteine in high-risk cardiac surgery patients. Crit Care Med 35: 1324– 1331, 2007 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

