p53 regulates metanephric development
- PMID: 19729440
- PMCID: PMC2799183
- DOI: 10.1681/ASN.2008121224
p53 regulates metanephric development
Abstract
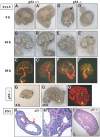
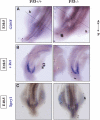
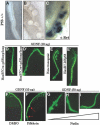
p53 is best known as a tumor suppressor that regulates cell-cycle, differentiation, and apoptosis pathways, but its potential role in embryonic development and organogenesis remains controversial. Here, p53(-/-) embryos bred on C57Bl6 background exhibited a spectrum of congenital abnormalities of the kidney and urinary tract, including ureteric bud (UB) ectopia, double ureters/collecting systems, delayed primary branching of the UB, and hypoplastic metanephroi. We observed ectopic UB outgrowth from the Wolffian duct (WD) in one third of p53(-/-) embryos. The prevalence of duplex was higher in embryos than in neonates, and ex vivo organ culture suggested that ectopic ureters can regress over time, leaving behind a dysplastic pole ("segmental dysgenesis"). Transgenic expression of dominant negative p53 or conditional inactivation of p53 in the UB but not in the metanephric mesenchyme lineage recapitulated the duplex phenotype. Mechanistically, p53 inactivation in the WD associated with enhanced sensitivity to glial cell line-derived neurotrophic factor (GDNF)-induced ectopic budding and potentiated phosphatidylinositol-3 kinase activation by GDNF in UB cells. Unlike several other models of UB ectopia, hypersensitivity of p53(-/-) WD to GDNF is not accompanied by reduced Sprouty-1 or anterior expansion of the GDNF domain. In summary, our data lend support for a restrictive role for p53 activity in UB outgrowth from the WD.
Figures




Similar articles
-
Protein kinase A regulates GDNF/RET-dependent but not GDNF/Ret-independent ureteric bud outgrowth from the Wolffian duct.Dev Biol. 2010 Nov 15;347(2):337-47. doi: 10.1016/j.ydbio.2010.08.029. Epub 2010 Sep 15. Dev Biol. 2010. PMID: 20816800 Free PMC article.
-
Role of Wnt5a-Ror2 signaling in morphogenesis of the metanephric mesenchyme during ureteric budding.Mol Cell Biol. 2014 Aug;34(16):3096-105. doi: 10.1128/MCB.00491-14. Epub 2014 Jun 2. Mol Cell Biol. 2014. PMID: 24891614 Free PMC article.
-
Tight regulation of p53 activity by Mdm2 is required for ureteric bud growth and branching.Dev Biol. 2011 May 15;353(2):354-66. doi: 10.1016/j.ydbio.2011.03.017. Epub 2011 Mar 21. Dev Biol. 2011. PMID: 21420949 Free PMC article.
-
GDNF and its receptors in the regulation of the ureteric branching.Int J Dev Biol. 1999;43(5):413-8. Int J Dev Biol. 1999. PMID: 10535317 Review.
-
GDNF/Ret signaling and the development of the kidney.Bioessays. 2006 Feb;28(2):117-27. doi: 10.1002/bies.20357. Bioessays. 2006. PMID: 16435290 Review.
Cited by
-
Molecular regulation of kidney development.Anat Cell Biol. 2013 Mar;46(1):19-31. doi: 10.5115/acb.2013.46.1.19. Epub 2013 Mar 25. Anat Cell Biol. 2013. PMID: 23560233 Free PMC article.
-
Duplex kidney formation: developmental mechanisms and genetic predisposition.F1000Res. 2020 Jan 6;9:F1000 Faculty Rev-2. doi: 10.12688/f1000research.19826.1. eCollection 2020. F1000Res. 2020. PMID: 32030122 Free PMC article. Review.
-
A p53-Pax2 pathway in kidney development: implications for nephrogenesis.PLoS One. 2012;7(9):e44869. doi: 10.1371/journal.pone.0044869. Epub 2012 Sep 12. PLoS One. 2012. PMID: 22984579 Free PMC article.
-
Mdm4 controls ureteric bud branching via regulation of p53 activity.Mech Dev. 2020 Sep;163:103616. doi: 10.1016/j.mod.2020.103616. Epub 2020 May 25. Mech Dev. 2020. PMID: 32464196 Free PMC article.
-
Renal stromal miRNAs are required for normal nephrogenesis and glomerular mesangial survival.Physiol Rep. 2015 Oct;3(10):e12537. doi: 10.14814/phy2.12537. Physiol Rep. 2015. PMID: 26438731 Free PMC article.
References
-
- Costantini F, Shakya R: GDNF/Ret signaling and the development of the kidney. Bioessays 28: 117–127, 2006 - PubMed
-
- Dressler GR: The cellular basis of kidney development. Annu Rev Cell Dev Biol 22: 509–529, 2006 - PubMed
-
- Towers PR, Woolf AS, Hardman P: Glial cell line-derived neurotrophic factor stimulates ureteric bud outgrowth and enhances survival of ureteric bud cells in vitro. Exp Nephrol 6: 337–351, 1998 - PubMed
-
- Herzlinger D: Inductive interactions during kidney development. Semin Nephrol 15: 255–262, 1995 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

