Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds
- PMID: 19729441
- PMCID: PMC2799178
- DOI: 10.1681/ASN.2008111196
Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds
Abstract
The scarcity of transplant allografts for diseased organs has prompted efforts at tissue regeneration using seeded scaffolds, an approach hampered by the enormity of cell types and complex architectures. Our goal was to decellularize intact organs in a manner that retained the matrix signal for differentiating pluripotent cells. We decellularized intact rat kidneys in a manner that preserved the intricate architecture and seeded them with pluripotent murine embryonic stem cells antegrade through the artery or retrograde through the ureter. Primitive precursor cells populated and proliferated within the glomerular, vascular, and tubular structures. Cells lost their embryonic appearance and expressed immunohistochemical markers for differentiation. Cells not in contact with the basement membrane matrix became apoptotic, thereby forming lumens. These observations suggest that the extracellular matrix can direct regeneration of the kidney, and studies using seeded scaffolds may help define differentiation pathways.
Figures

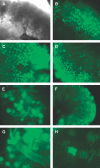
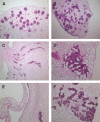
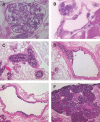
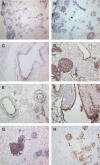

References
-
- Lanza R, Langer R, Vicanti J: Principles of Tissue Engineering San Diego, Academic Press, 2000
-
- Atala A: Recent applications of regenerative medicine to urologic structures and related tissues. Curr Opin Urol 16: 305–309, 2006 - PubMed
-
- Gilbert TW, Sellaro TL, Badylak SF: Decellularization of tissues and organs. Biomaterials 27: 3675–3683, 2006 - PubMed
-
- Matsunuma H, Kagami H, Narita Y, Hata K, Ono Y, Ohshima S, Ueda M: Constructing a tissue-engineered ureter using a decellularized matrix with cultured uroepithelial cells and bone marrow-derived mononuclear cells. Tissue Eng 12: 509–518, 2006 - PubMed
-
- Grauss RW, Hazekamp M, Oppenhuizen F, Munsteren C, Gittenberger-de Groot A, DeRuiter M: Histological evaluation of decellularised porcine aortic valves: Matrix changes due to different decellularisation methods. Eur J Cardiothorac Surg 27: 566–571, 2005 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

