Alterations in aortic cellular constituents during thoracic aortic aneurysm development: myofibroblast-mediated vascular remodeling
- PMID: 19729479
- PMCID: PMC2751569
- DOI: 10.2353/ajpath.2009.081141
Alterations in aortic cellular constituents during thoracic aortic aneurysm development: myofibroblast-mediated vascular remodeling
Abstract
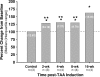
The present study tested the hypothesis that changes in the resident endogenous cellular population accompany alterations in aortic collagen and elastin content during thoracic aortic aneurysm (TAA) development in a murine model. Descending thoracic aortas were analyzed at various time points (2, 4, 8, and 16 weeks) post-TAA induction (0.5 M CaCl2, 15 minutes). Aortic tissue sections were subjected to histological staining and morphometric analysis for collagen and elastin, as well as immunostaining for cell-type-specific markers to quantify fibroblasts, myofibroblasts, and smooth-muscle cells. Results were compared with reference control mice processed in the same fashion. Aortic dilatation was accompanied by changes in the elastic architecture that included: a decreased number of elastic lamellae (from 6 to 4); altered area fraction of elastin (elevated at 4 weeks and decreased at 16 weeks); and a decreased area between elastic lamellae (minimum reached at 4 weeks). Total collagen content did not change over time. Increased immunoreactivity for fibroblast and myofibroblast markers was observed at 8- and 16-week post-TAA-induction, whereas immunoreactivity for smooth-muscle cell markers peaked at 4 weeks and returned to baseline by 16 weeks. Therefore, this study demonstrated that changes in aortic elastin content were accompanied by the emergence of a subset of fibroblast-derived myofibroblasts whose altered phenotype may play a significant role in TAA development through the enhancement of extracellular matrix proteolysis.
Figures
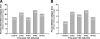

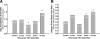




References
-
- Coady MA, Rizzo JA, Goldstein LJ, Elefteriades JA. Natural history, pathogenesis, and etiology of thoracic aortic aneurysms and dissections. Cardiol Clin. 1999;17:615–635. - PubMed
-
- Isselbacher EM. Thoracic and abdominal aortic aneurysms. Circulation. 2005;111:816–828. - PubMed
-
- Barbour JR, Spinale FG, Ikonomidis JS. Proteinase systems and thoracic aortic aneurysm progression. J Surg Res. 2007;139:292–307. - PubMed
-
- Koullias GJ, Ravichandran P, Korkolis DP, Rimm DL, Elefteriades JA. Increased tissue microarray matrix metalloproteinase expression favors proteolysis in thoracic aortic aneurysms and dissections. Ann Thorac Surg. 2004;78:2106–2110. discussion 2110–2101. - PubMed
-
- Dingemans KP, Teeling P, Lagendijk JH, Becker AE. Extracellular matrix of the human aortic media: an ultrastructural histochemical and immunohistochemical study of the adult aortic media. Anat Rec. 2000;258:1–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

