Comparative analysis of metastasis variants derived from human prostate carcinoma cells: roles in intravasation of VEGF-mediated angiogenesis and uPA-mediated invasion
- PMID: 19729488
- PMCID: PMC2751560
- DOI: 10.2353/ajpath.2009.090384
Comparative analysis of metastasis variants derived from human prostate carcinoma cells: roles in intravasation of VEGF-mediated angiogenesis and uPA-mediated invasion
Abstract
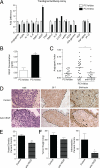
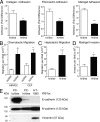
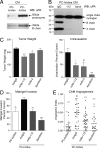
To analyze the process of tumor cell intravasation, we used the human tumor-chick embryo spontaneous metastasis model to select in vivo high (PC-hi/diss) and low (PC-lo/diss) disseminating variants from the human PC-3 prostate carcinoma cell line. These variants dramatically differed in their intravasation and dissemination capacities in both chick embryo and mouse spontaneous metastasis models. Concomitant with enhanced intravasation, PC-hi/diss exhibited increased angiogenic potential in avian and murine models. PC-hi/diss angiogenesis and intravasation were dependent on increased secretion of vascular endothelial growth factor (VEGF), since treating developing tumors with a function-blocking anti-VEGF antibody simultaneously inhibited both processes without affecting primary tumor growth. PC-hi/diss cells were also more migratory and invasive, suggestive of heightened ability to escape from primary tumors due to matrix-degrading activity. Consistent with this suggestion, PC-hi/diss cells produced more of the serine protease urokinase-type plasminogen activator (uPA) as compared with PC-lo/diss. The functional role of uPA in PC-hi/diss dissemination was confirmed by inhibition of invasion, angiogenesis, and intravasation with specific function-blocking antibodies that prevented uPA activation and blocked uPA activity. These processes were similarly sensitive to aprotinin, a potent inhibitor of serine proteases, including uPA-generated plasmin. Thus, our comparison of the PC-3 intravasation variants points to key roles for the uPA-plasmin system in PC-hi/diss intravasation, possibly via (1) promoting tumor cell matrix invasion and (2) facilitating development of VEGF-dependent angiogenic blood vessels.
Figures

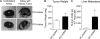


Similar articles
-
Activation of pro-uPA is critical for initial escape from the primary tumor and hematogenous dissemination of human carcinoma cells.Neoplasia. 2011 Sep;13(9):806-21. doi: 10.1593/neo.11704. Neoplasia. 2011. PMID: 21969814 Free PMC article.
-
Activity-based protein profiling implicates urokinase activation as a key step in human fibrosarcoma intravasation.J Biol Chem. 2006 Jun 9;281(23):15997-6005. doi: 10.1074/jbc.M601223200. Epub 2006 Apr 12. J Biol Chem. 2006. PMID: 16611636
-
Tumor MMP-1 activates endothelial PAR1 to facilitate vascular intravasation and metastatic dissemination.Cancer Res. 2013 Jul 15;73(14):4196-211. doi: 10.1158/0008-5472.CAN-12-4495. Epub 2013 May 16. Cancer Res. 2013. PMID: 23687338 Free PMC article.
-
[Mechanism of tumor cell-induced extracellular matrix degradation--inhibition of cell-surface proteolytic activity might have a therapeutic effect on tumor cell invasion and metastasis].Nihon Sanka Fujinka Gakkai Zasshi. 1996 Aug;48(8):623-32. Nihon Sanka Fujinka Gakkai Zasshi. 1996. PMID: 8808830 Review. Japanese.
-
Urokinase-type plasminogen activator (uPA) and its receptor (CD87): a new target in tumor invasion and metastasis.J Obstet Gynaecol (Tokyo 1995). 1995 Apr;21(2):151-65. doi: 10.1111/j.1447-0756.1995.tb01089.x. J Obstet Gynaecol (Tokyo 1995). 1995. PMID: 8556577 Review.
Cited by
-
Blocking of CDCP1 cleavage in vivo prevents Akt-dependent survival and inhibits metastatic colonization through PARP1-mediated apoptosis of cancer cells.Oncogene. 2012 Aug 30;31(35):3924-38. doi: 10.1038/onc.2011.555. Epub 2011 Dec 19. Oncogene. 2012. PMID: 22179830 Free PMC article.
-
Microfluidic Applications in Prostate Cancer Research.Micromachines (Basel). 2024 Sep 27;15(10):1195. doi: 10.3390/mi15101195. Micromachines (Basel). 2024. PMID: 39459070 Free PMC article. Review.
-
Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation.Am J Pathol. 2011 Sep;179(3):1455-70. doi: 10.1016/j.ajpath.2011.05.031. Epub 2011 Jul 8. Am J Pathol. 2011. PMID: 21741942 Free PMC article.
-
LTBP3 promotes early metastatic events during cancer cell dissemination.Oncogene. 2018 Apr;37(14):1815-1829. doi: 10.1038/s41388-017-0075-1. Epub 2018 Jan 19. Oncogene. 2018. PMID: 29348457 Free PMC article.
-
CD44 enhances invasion of basal-like breast cancer cells by upregulating serine protease and collagen-degrading enzymatic expression and activity.Breast Cancer Res. 2012 May 23;14(3):R84. doi: 10.1186/bcr3199. Breast Cancer Res. 2012. PMID: 22621373 Free PMC article.
References
-
- Naito S, Walker SM, Fidler IJ. In vivo selection of human renal cell carcinoma cells with high metastatic potential in nude mice. Clin Exp Metastasis. 1989;7:381–389. - PubMed
-
- Vezeridis MP, Tzanakakis GN, Meitner PA, Doremus CM, Tibbetts LM, Calabresi P. In vivo selection of a highly metastatic cell line from a human pancreatic carcinoma in the nude mouse. Cancer. 1992;69:2060–2063. - PubMed
-
- Pettaway CA, Pathak S, Greene G, Ramirez E, Wilson MR, Killion JJ, Fidler IJ. Selection of highly metastatic variants of different human prostatic carcinomas using orthotopic implantation in nude mice. Clin Cancer Res. 1996;2:1627–1636. - PubMed
-
- Morikawa K, Walker SM, Jessup JM, Fidler IJ. In vivo selection of highly metastatic cells from surgical specimens of different primary human colon carcinomas implanted into nude mice. Cancer Res. 1988;48:1943–1948. - PubMed
-
- Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

