Neuroimaging in superficial siderosis: an in-depth look
- PMID: 19729538
- PMCID: PMC7964065
- DOI: 10.3174/ajnr.A1628
Neuroimaging in superficial siderosis: an in-depth look
Abstract
Despite extensive imaging, a source of bleeding is often not evident during the evaluation of patients with superficial siderosis (SS) of the central nervous system. An intraspinal fluid-filled collection of variable dimensions is frequently seen on spine MR imaging in patients with idiopathic SS. A similar finding has also been reported in patients with craniospinal hypotension. This review discusses the role of multitechnique imaging in the work-up of patients with SS and focuses on recent developments.
Figures
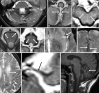






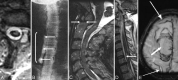
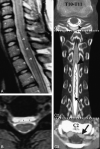
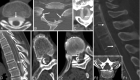

Comment in
-
Superficial siderosis in cerebral amyloid angiopathy.AJNR Am J Neuroradiol. 2010 Feb;31(2):E29. doi: 10.3174/ajnr.A1913. Epub 2009 Oct 22. AJNR Am J Neuroradiol. 2010. PMID: 19850768 Free PMC article. No abstract available.
-
Further in-depth look at superficial siderosis (and intracranial hypotension).AJNR Am J Neuroradiol. 2010 Sep;31(8):E72; author reply E73. doi: 10.3174/ajnr.A2172. Epub 2010 Jun 25. AJNR Am J Neuroradiol. 2010. PMID: 20581071 Free PMC article. No abstract available.
References
-
- Fearnley JM, Stevens JM, Rudge P. Superficial siderosis of the central nervous system. Brain 1995; 118: 1051–66 - PubMed
-
- Kumar N, Cohen-Gadol AA, Wright RA, et al. . Superficial siderosis. Neurology 2006; 66: 1144–52 - PubMed
-
- Kumar N. Superficial siderosis: associations and therapeutic implications. Arch Neurol 2007; 64: 491–96 - PubMed
-
- Koeppen AH, Michael SC, Li D, et al. . The pathology of superficial siderosis of the central nervous system. Acta Neuropathol 2008; 116: 371–82. Epub 2008 Aug 12 - PubMed
-
- Koeppen AH, Dentinger MP. Brain hemosiderin and superficial siderosis of the central nervous system. J Neuropathol Exp Neurol 1988; 47: 249–70 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
