The Hedgehog transcription factor Gli3 modulates angiogenesis
- PMID: 19729595
- PMCID: PMC3175353
- DOI: 10.1161/CIRCRESAHA.109.206706
The Hedgehog transcription factor Gli3 modulates angiogenesis
Abstract
Rationale: The Gli transcription factors are mediators of Hedgehog (Hh) signaling and have been shown to play critical roles during embryogenesis. Previously, we have demonstrated that the Hh pathway is reactivated by ischemia in adult mammals, and that this pathway can be stimulated for therapeutic benefit; however, the specific roles of the Gli transcription factors during ischemia-induced Hh signaling have not been elucidated.
Objective: To investigate the role of Gli3 in ischemic tissue repair.
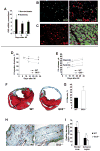
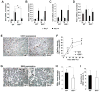
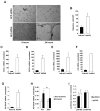
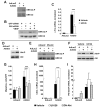
Methods and results: Gli3-haploinsufficient (Gli3(+/-)) mice and their wild-type littermates were physiologically similar in the absence of ischemia; however, histological assessments of capillary density and echocardiographic measurements of left ventricular ejection fractions were reduced in Gli3(+/-) mice compared to wild-type mice after surgically induced myocardial infarction, and fibrosis was increased. Gli3-deficient mice also displayed reduced capillary density after induction of hindlimb ischemia and an impaired angiogenic response to vascular endothelial growth factor in the corneal angiogenesis model. In endothelial cells, adenovirus-mediated overexpression of Gli3 promoted migration (modified Boyden chamber), small interfering RNA-mediated downregulation of Gli3 delayed tube formation (Matrigel), and Western analyses identified increases in Akt phosphorylation, extracellular signal-regulated kinase (ERK)1/2 activation, and c-Fos expression; however, promoter-reporter assays indicated that Gli3 overexpression does not modulate Gli-dependent transcription. Furthermore, the induction of endothelial cell migration by Gli3 was dependent on Akt and ERK1/2 activation.
Conclusions: Collectively, these observations indicate that Gli3 contributes to vessel growth under both ischemic and nonischemic conditions and provide the first evidence that Gli3 regulates angiogenesis and endothelial cell activity in adult mammals.
Conflict of interest statement
Figures

Similar articles
-
Interaction between hedgehog signalling and PAX6 dosage mediates maintenance and regeneration of the corneal epithelium.Mol Vis. 2012;18:139-50. Epub 2012 Jan 18. Mol Vis. 2012. PMID: 22275805 Free PMC article.
-
Gli3 regulation of myogenesis is necessary for ischemia-induced angiogenesis.Circ Res. 2013 Oct 25;113(10):1148-58. doi: 10.1161/CIRCRESAHA.113.301546. Epub 2013 Sep 17. Circ Res. 2013. PMID: 24044950 Free PMC article.
-
Sonic hedgehog induces angiogenesis via Rho kinase-dependent signaling in endothelial cells.J Mol Cell Cardiol. 2010 Sep;49(3):490-8. doi: 10.1016/j.yjmcc.2010.05.003. Epub 2010 May 15. J Mol Cell Cardiol. 2010. PMID: 20478312 Free PMC article.
-
A proteomic approach identifies SAFB-like transcription modulator (SLTM) as a bidirectional regulator of GLI family zinc finger transcription factors.J Biol Chem. 2019 Apr 5;294(14):5549-5561. doi: 10.1074/jbc.RA118.007018. Epub 2019 Feb 19. J Biol Chem. 2019. PMID: 30782847 Free PMC article.
-
Suppressor of fused controls mid-hindbrain patterning and cerebellar morphogenesis via GLI3 repressor.J Neurosci. 2011 Feb 2;31(5):1825-36. doi: 10.1523/JNEUROSCI.2166-10.2011. J Neurosci. 2011. PMID: 21289193 Free PMC article.
Cited by
-
Mesenchymal stem cell therapy for attenuation of scar formation during wound healing.Stem Cell Res Ther. 2012 May 31;3(3):20. doi: 10.1186/scrt111. Stem Cell Res Ther. 2012. PMID: 22668751 Free PMC article. Review.
-
Hh signaling in regeneration of the ischemic heart.Cell Mol Life Sci. 2017 Oct;74(19):3481-3490. doi: 10.1007/s00018-017-2534-9. Epub 2017 May 18. Cell Mol Life Sci. 2017. PMID: 28523343 Free PMC article. Review.
-
Metformin alleviates hyperglycemia-induced endothelial impairment by downregulating autophagy via the Hedgehog pathway.Autophagy. 2019 May;15(5):843-870. doi: 10.1080/15548627.2019.1569913. Epub 2019 Jan 27. Autophagy. 2019. PMID: 30653446 Free PMC article.
-
End binding-3 inhibitor activates regenerative program in age-related macular degeneration.Cell Rep Med. 2023 Oct 17;4(10):101223. doi: 10.1016/j.xcrm.2023.101223. Epub 2023 Oct 3. Cell Rep Med. 2023. PMID: 37794584 Free PMC article.
-
Hedgehog signalling regulates liver sinusoidal endothelial cell capillarisation.Gut. 2013 Feb;62(2):299-309. doi: 10.1136/gutjnl-2011-301494. Epub 2012 Feb 23. Gut. 2013. PMID: 22362915 Free PMC article.
References
-
- Sasaki H, Nishizaki Y, Hui C, Nakafuku M, Kondoh H. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development. 1999;126:3915–3924. - PubMed
-
- Hui CC, Slusarski D, Platt KA, Holmgren R, Joyner AL. Expression of three mouse homologs of the Drosophila segment polarity gene cubitus interruptus, Gli, Gli-2, and Gli-3, in ectoderm- and mesoderm-derived tissues suggests multiple roles during postimplantation development. Dev Biol. 1994;162:402–413. - PubMed
-
- Walterhouse D, Ahmed M, Slusarski D, Kalamaras J, Boucher D, Holmgren R, Iannaccone P. gli, a zinc finger transcription factor and oncogene, is expressed during normal mouse development. Dev Dyn. 1993;196:91–102. - PubMed
-
- Litingtung Y, Chiang C. Specification of ventral neuron types is mediated by an antagonistic interaction between Shh and Gli3. Nat Neurosci. 2000;3:979–985. - PubMed
-
- Rallu M, Machold R, Gaiano N, Corbin JG, McMahon AP, Fishell G. Dorsoventral patterning is established in the telencephalon of mutants lacking both Gli3 and Hedgehog signaling. Development. 2002;129:4963–4974. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

