Short-course therapy with daily rifapentine in a murine model of latent tuberculosis infection
- PMID: 19729664
- PMCID: PMC2784419
- DOI: 10.1164/rccm.200905-0795OC
Short-course therapy with daily rifapentine in a murine model of latent tuberculosis infection
Abstract
Rationale: Regimens recommended to treat latent tuberculosis infection (LTBI) are 3 to 9 months long. A 2-month rifampin+pyrazinamide regimen is no longer recommended. Shorter regimens are highly desirable. Because substituting rifapentine for rifampin in the standard regimen for active tuberculosis halves the treatment duration needed to prevent relapse in mice, we hypothesized daily rifapentine-based regimens could shorten LTBI treatment to 2 months or less.
Objectives: To improve an existing model of LTBI chemotherapy and evaluate the efficacy of daily rifapentine-based regimens.
Methods: Mice were immunized with a more immunogenic recombinant Bacille Calmette-Guérin strain (rBCG30) and received very low-dose aerosol infection with Mycobacterium tuberculosis to establish a stable lung bacterial burden below 10(4) CFU without drug treatment. Mice received a control (isoniazid alone, rifampin alone, rifampin+isoniazid, rifampin+pyrazinamide) or test (rifapentine alone, rifapentine+isoniazid, rifapentine+pyrazinamide, rifapentine+isoniazid+pyrazinamide) regimen for 8 weeks. Rifamycin doses were 10 mg/kg/d, analogous to the same human doses. Outcomes were biweekly lung CFU counts and relapse after 4 to 8 weeks of treatment.
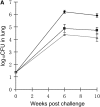
Measurements and main results: M. tuberculosis CFU counts remained stable around 3.65 log(10) in immunized, untreated mice. Isoniazid or rifampin left all or most mice culture-positive at week 8. Rifampin+isoniazid cured 0 and 53% of mice and rifampin+pyrazinamide cured 47 and 100% of mice in 4 and 8 weeks, respectively. Rifapentine-based regimens were more active than rifampin+isoniazid and indistinguishable from rifampin+pyrazinamide.
Conclusions: In this improved murine model of LTBI chemotherapy with very low lung burden, existing regimens were well represented. Daily rifapentine-based regimens were at least as active as rifampin+pyrazinamide, suggesting they could effectively treat LTBI in 6 to 8 weeks.
Figures


References
-
- Centers for Disease Control and Prevention and American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Morb Mortal Wkly Rep 2000;49:1–51. - PubMed
-
- Broekmans JF, Migliori GB, Rieder HL, Lees J, Ruutu P, Loddenkemper R, Raviglione MC, World Health Organization. European framework for tuberculosis control and elimination in countries with a low incidence: recommendations of the World Health Organization (WHO), International Union Against Tuberculosis and Lung Disease (IUATLD) and Royal Netherlands Tuberculosis Association (KNCV) Working Group. Eur Respir J 2002;19:765–775. - PubMed
-
- Mack U, Migliori GB, Sester M, Rieder HL, Ehlers S, Goletti D, Bossink A, Magdorf K, Holscher C, Kampmann B, et al. LTBI: latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statement. Eur Respir J 2009;33:956–973. - PubMed
-
- Public Health Agency of Canada and Canadian Lung Association/Canadian Thoracic Association. Canadian tuberculosis standards, 6th ed. Ottowa: Ministry of Health; 2007. Cat no. HP40-18/2007E.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

