Coadaptation of Helicobacter pylori and humans: ancient history, modern implications
- PMID: 19729845
- PMCID: PMC2735910
- DOI: 10.1172/JCI38605
Coadaptation of Helicobacter pylori and humans: ancient history, modern implications
Abstract
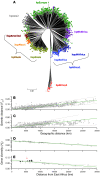
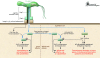
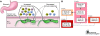
Humans have been colonized by Helicobacter pylori for at least 50,000 years and probably throughout their evolution. H. pylori has adapted to humans, colonizing children and persisting throughout life. Most strains possess factors that subtly modulate the host environment, increasing the risk of peptic ulceration, gastric adenocarcinoma, and possibly other diseases. H. pylori genes encoding these and other factors rapidly evolve through mutation and recombination, changing the bacteria-host interaction. Although immune and physiologic responses to H. pylori also contribute to pathogenesis, humans have evolved in concert with the bacterium, and its recent absence throughout the life of many individuals has led to new human physiological changes. These may have contributed to recent increases in esophageal adenocarcinoma and, more speculatively, other modern diseases.
Figures



References
-
- Banatvala N., et al. The cohort effect and Helicobacter pylori. J. Infect. Dis. 1993;168:219–221. - PubMed

