The impact of malaria parasitism: from corpuscles to communities
- PMID: 19729847
- PMCID: PMC2735907
- DOI: 10.1172/JCI38307
The impact of malaria parasitism: from corpuscles to communities
Abstract
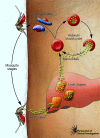
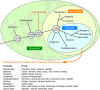
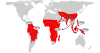
Malaria continues to exert a tremendous health burden on human populations, reflecting astonishingly successful adaptations of the causative Plasmodium parasites. We discuss here how this burden has driven the natural selection of numerous polymorphisms in the genes encoding hemoglobin and other erythrocyte proteins and some effectors of immunity. Plasmodium falciparum, the most deadly parasite species in humans, displays a vigorous system of antigen variation to counter host defenses and families of functionally redundant ligands to invade human cells. Advances in genetics and genomics are providing fresh insights into the nature of these evolutionary adaptations, processes of parasite transmission and infection, and the difficult challenges of malaria control.
Figures


References
-
- WHO. 2008. World malaria report. World Health Organization Press. Geneva, Switzerland. http://apps.who.int/malaria/wmr2008/malaria2008.pdf .
-
- Okamoto N., McFadden G.I. The mother of all parasites. Future Microbiol. 2008;3:391–395. doi: 10.2217/17460913.3.4.391. - DOI

