Restraint of apoptosis during mitosis through interdomain phosphorylation of caspase-2
- PMID: 19730412
- PMCID: PMC2771089
- DOI: 10.1038/emboj.2009.253
Restraint of apoptosis during mitosis through interdomain phosphorylation of caspase-2
Abstract
The apoptotic initiator caspase-2 has been implicated in oocyte death, in DNA damage- and heat shock-induced death, and in mitotic catastrophe. We show here that the mitosis-promoting kinase, cdk1-cyclin B1, suppresses apoptosis upstream of mitochondrial cytochrome c release by phosphorylating caspase-2 within an evolutionarily conserved sequence at Ser 340. Phosphorylation of this residue, situated in the caspase-2 interdomain, prevents caspase-2 activation. S340 was susceptible to phosphatase 1 dephosphorylation, and an interaction between phosphatase 1 and caspase-2 detected during interphase was lost in mitosis. Expression of S340A non-phosphorylatable caspase-2 abrogated mitotic suppression of caspase-2 and apoptosis in various settings, including oocytes induced to undergo cdk1-dependent maturation. Moreover, U2OS cells treated with nocodazole were found to undergo mitotic catastrophe more readily when endogenous caspase-2 was replaced with the S340A mutant to lift mitotic inhibition. These data demonstrate that for apoptotic stimuli transduced by caspase-2, cell death is prevented during mitosis through the inhibitory phosphorylation of caspase-2 and suggest that under conditions of mitotic arrest, cdk1-cyclin B1 activity must be overcome for apoptosis to occur.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
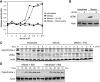

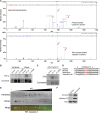
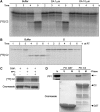


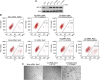

Similar articles
-
Phosphorylation of caspase-9 by CDK1/cyclin B1 protects mitotic cells against apoptosis.Mol Cell. 2007 Apr 27;26(2):301-10. doi: 10.1016/j.molcel.2007.03.019. Mol Cell. 2007. PMID: 17466630
-
Role of cyclin B1/Cdc2 in mediating Bcl-XL phosphorylation and apoptotic cell death following nocodazole-induced mitotic arrest.Mol Carcinog. 2014 Feb;53(2):125-37. doi: 10.1002/mc.21956. Epub 2012 Sep 4. Mol Carcinog. 2014. PMID: 22949227
-
Generation of an indestructible cyclin B1 by caspase-6-dependent cleavage during mitotic catastrophe.Oncogene. 2009 Jan 15;28(2):170-83. doi: 10.1038/onc.2008.369. Epub 2008 Sep 29. Oncogene. 2009. PMID: 18820706
-
Cell death by mitotic catastrophe: a molecular definition.Oncogene. 2004 Apr 12;23(16):2825-37. doi: 10.1038/sj.onc.1207528. Oncogene. 2004. PMID: 15077146 Review.
-
Cyclin-dependent kinase-1: linking apoptosis to cell cycle and mitotic catastrophe.Cell Death Differ. 2002 Dec;9(12):1287-93. doi: 10.1038/sj.cdd.4401130. Cell Death Differ. 2002. PMID: 12478465 Review.
Cited by
-
The p53-caspase-2 axis in the cell cycle and DNA damage response.Exp Mol Med. 2021 Apr;53(4):517-527. doi: 10.1038/s12276-021-00590-2. Epub 2021 Apr 14. Exp Mol Med. 2021. PMID: 33854186 Free PMC article. Review.
-
Phosphorylation by Aurora B kinase regulates caspase-2 activity and function.Cell Death Differ. 2021 Jan;28(1):349-366. doi: 10.1038/s41418-020-00604-y. Epub 2020 Aug 18. Cell Death Differ. 2021. PMID: 32811973 Free PMC article.
-
Validation of the Intermolecular Disulfide Bond in Caspase-2.Biology (Basel). 2024 Jan 17;13(1):49. doi: 10.3390/biology13010049. Biology (Basel). 2024. PMID: 38248479 Free PMC article.
-
Bim vanishes in the light of a mitotic Aurora.Cell Death Differ. 2013 Dec;20(12):1597-8. doi: 10.1038/cdd.2013.140. Cell Death Differ. 2013. PMID: 24212928 Free PMC article. No abstract available.
-
Caspase 2 in mitotic catastrophe: The terminator of aneuploid and tetraploid cells.Mol Cell Oncol. 2017 Mar 10;4(3):e1299274. doi: 10.1080/23723556.2017.1299274. eCollection 2017. Mol Cell Oncol. 2017. PMID: 28616577 Free PMC article.
References
-
- Allan LA, Clarke PR (2007) Phosphorylation of caspase-9 by CDK1/cyclin B1 protects mitotic cells against apoptosis. Mol Cell 26: 301–310 - PubMed
-
- Allan LA, Morrice N, Brady S, Magee G, Pathak S, Clarke PR (2003) Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK MAPK. Nat Cell Biol 5: 647–654 - PubMed
-
- Baliga BC, Read SH, Kumar S (2004) The biochemical mechanism of caspase-2 activation. Cell Death Differ 11: 1234–1241 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

