Inositol 1,4,5-trisphosphate signalling regulates the avoidance response to nose touch in Caenorhabditis elegans
- PMID: 19730689
- PMCID: PMC2729924
- DOI: 10.1371/journal.pgen.1000636
Inositol 1,4,5-trisphosphate signalling regulates the avoidance response to nose touch in Caenorhabditis elegans
Abstract
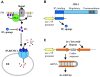
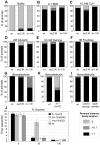
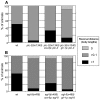
When Caenorhabditis elegans encounters an unfavourable stimulus at its anterior, it responds by initiating an avoidance response, namely reversal of locomotion. The amphid neurons, ASHL and ASHR, are polymodal in function, with roles in the avoidance responses to high osmolarity, nose touch, and both volatile and non-volatile repellents. The mechanisms that underlie the ability of the ASH neurons to respond to such a wide range of stimuli are still unclear. We demonstrate that the inositol 1,4,5-trisphosphate receptor (IP(3)R), encoded by itr-1, functions in the reversal responses to nose touch and benzaldehyde, but not in other known ASH-mediated responses. We show that phospholipase Cbeta (EGL-8) and phospholipase Cgamma (PLC-3), which catalyse the production of IP(3), both function upstream of ITR-1 in the response to nose touch. We use neuron-specific gene rescue and neuron-specific disruption of protein function to show that the site of ITR-1 function is the ASH neurons. By rescuing plc-3 and egl-8 in a neuron-specific manner, we show that both are acting in ASH. Imaging of nose touch-induced Ca(2+) transients in ASH confirms these conclusions. In contrast, the response to benzaldehyde is independent of PLC function. Thus, we have identified distinct roles for the IP(3)R in two specific responses mediated by ASH.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- White JG, Southgate E, Thomson JN, Brenner S. The Structure of the Nervous System of the Nematode Caenorhabditis elegans. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 1986;314:1–340. - PubMed
-
- Bargmann CI, Thomas JH, Horvitz HR. Chemosensory cell function in the behavior and development of Caenorhabditis elegans. Cold Spring Harb Symp Quant Biol. 1990;55:529–538. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

