CD8(+) T cells restrict Yersinia pseudotuberculosis infection: bypass of anti-phagocytosis by targeting antigen-presenting cells
- PMID: 19730693
- PMCID: PMC2731216
- DOI: 10.1371/journal.ppat.1000573
CD8(+) T cells restrict Yersinia pseudotuberculosis infection: bypass of anti-phagocytosis by targeting antigen-presenting cells
Abstract
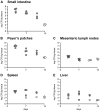
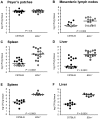
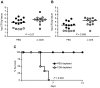
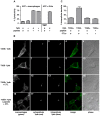
All Yersinia species target and bind to phagocytic cells, but uptake and destruction of bacteria are prevented by injection of anti-phagocytic Yop proteins into the host cell. Here we provide evidence that CD8(+) T cells, which canonically eliminate intracellular pathogens, are important for restricting Yersinia, even though bacteria are primarily found in an extracellular locale during the course of disease. In a model of infection with attenuated Y. pseudotuberculosis, mice deficient for CD8(+) T cells were more susceptible to infection than immunocompetent mice. Although exposure to attenuated Y. pseudotuberculosis generated T(H)1-type antibody responses and conferred protection against challenge with fully virulent bacteria, depletion of CD8(+) T cells during challenge severely compromised protective immunity. Strikingly, mice lacking the T cell effector molecule perforin also succumbed to Y. pseudotuberculosis infection. Given that the function of perforin is to kill antigen-presenting cells, we reasoned that cell death marks bacteria-associated host cells for internalization by neighboring phagocytes, thus allowing ingestion and clearance of the attached bacteria. Supportive of this model, cytolytic T cell killing of Y. pseudotuberculosis-associated host cells results in engulfment by neighboring phagocytes of both bacteria and target cells, bypassing anti-phagocytosis. Our findings are consistent with a novel function for cell-mediated immune responses protecting against extracellular pathogens like Yersinia: perforin and CD8(+) T cells are critical for hosts to overcome the anti-phagocytic action of Yops.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Brubaker RR. The genus Yersinia: biochemistry and genetics of virulence. Curr Top Microbiol Immunol. 1972;57:111–158. - PubMed
-
- Prentice MB, Rahalison L. Plague. Lancet. 2007;369:1196–1207. - PubMed
-
- Naktin J, Beavis KG. Yersinia enterocolitica and Yersinia pseudotuberculosis. Clin Lab Med. 1999;19:vi. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI056068/HHMI/Howard Hughes Medical Institute/United States
- R29 AI039558/AI/NIAID NIH HHS/United States
- P30DK34928/DK/NIDDK NIH HHS/United States
- PF0325701MBC/MB/BHP HRSA HHS/United States
- R37AI23538/HHMI/Howard Hughes Medical Institute/United States
- R01 AI039558/AI/NIAID NIH HHS/United States
- AI055962/HHMI/Howard Hughes Medical Institute/United States
- AI39558/HHMI/Howard Hughes Medical Institute/United States
- R37 AI023538/AI/NIAID NIH HHS/United States
- P30 DK034928/DK/NIDDK NIH HHS/United States
- R01 AI055962/AI/NIAID NIH HHS/United States
- R01 AI056068/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

