Homeostatic plasticity of striatal neurons intrinsic excitability following dopamine depletion
- PMID: 19730738
- PMCID: PMC2733153
- DOI: 10.1371/journal.pone.0006908
Homeostatic plasticity of striatal neurons intrinsic excitability following dopamine depletion
Abstract
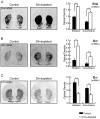
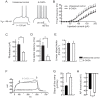
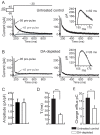
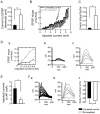
The striatum is the major input structure of basal ganglia and is involved in adaptive control of behaviour through the selection of relevant informations. Dopaminergic neurons that innervate striatum die in Parkinson disease, leading to inefficient adaptive behaviour. Neuronal activity of striatal medium spiny neurons (MSN) is modulated by dopamine receptors. Although dopamine signalling had received substantial attention, consequences of dopamine depletion on MSN intrinsic excitability remain unclear. Here we show, by performing perforated patch clamp recordings on brain slices, that dopamine depletion leads to an increase in MSN intrinsic excitability through the decrease of an inactivating A-type potassium current, I(A). Despite the large decrease in their excitatory synaptic inputs determined by the decreased dendritic spines density and the increase in minimal current to evoke the first EPSP, this increase in intrinsic excitability resulted in an enhanced responsiveness to their remaining synapses, allowing them to fire similarly or more efficiently following input stimulation than in control condition. Therefore, this increase in intrinsic excitability through the regulation of I(A) represents a form of homeostatic plasticity allowing neurons to compensate for perturbations in synaptic transmission and to promote stability in firing. The present observations show that this homeostatic ability to maintain firing rates within functional range also occurs in pathological conditions, allowing stabilizing neural computation within affected neuronal networks.
Conflict of interest statement
Figures


References
-
- Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science. 1994;265:1826–1831. - PubMed
-
- Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. - PubMed
-
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. - PubMed
-
- Calabresi P, Centonze D, Bernardi G. Electrophysiology of dopamine in normal and denervated striatal neurons. Trends Neurosci. 2000;23:S57–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

