Relative roles of TGF-beta1 and Wnt in the systemic regulation and aging of satellite cell responses
- PMID: 19732043
- PMCID: PMC2783265
- DOI: 10.1111/j.1474-9726.2009.00517.x
Relative roles of TGF-beta1 and Wnt in the systemic regulation and aging of satellite cell responses
Abstract
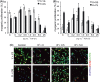
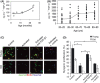
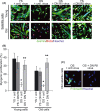
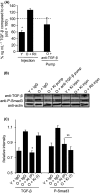
Muscle stem (satellite) cells are relatively resistant to cell-autonomous aging. Instead, their endogenous signaling profile and regenerative capacity is strongly influenced by the aged P-Smad3, differentiated niche, and by the aged circulation. With respect to muscle fibers, we previously established that a shift from active Notch to excessive transforming growth factor-beta (TGF-beta) induces CDK inhibitors in satellite cells, thereby interfering with productive myogenic responses. In contrast, the systemic inhibitor of muscle repair, elevated in old sera, was suggested to be Wnt. Here, we examined the age-dependent myogenic activity of sera TGF-beta1, and its potential cross-talk with systemic Wnt. We found that sera TGF-beta1 becomes elevated within aged humans and mice, while systemic Wnt remained undetectable in these species. Wnt also failed to inhibit satellite cell myogenicity, while TGF-beta1 suppressed regenerative potential in a biphasic fashion. Intriguingly, young levels of TGF-beta1 were inhibitory and young sera suppressed myogenesis if TGF-beta1 was activated. Our data suggest that platelet-derived sera TGF-beta1 levels, or endocrine TGF-beta1 levels, do not explain the age-dependent inhibition of muscle regeneration by this cytokine. In vivo, TGF-beta neutralizing antibody, or a soluble decoy, failed to reduce systemic TGF-beta1 and rescue myogenesis in old mice. However, muscle regeneration was improved by the systemic delivery of a TGF-beta receptor kinase inhibitor, which attenuated TGF-beta signaling in skeletal muscle. Summarily, these findings argue against the endocrine path of a TGF-beta1-dependent block on muscle regeneration, identify physiological modalities of age-imposed changes in TGF-beta1, and introduce new therapeutic strategies for the broad restoration of aged organ repair.
Figures


References
-
- Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal. Biochem. 1994;216:276–284. - PubMed
-
- Andrews ZB, Zhao H, Frugier T, Meguro R, Grattan DR, Koishi K, McLennan IS. Transforming growth factor beta2 haploinsufficient mice develop age-related nigrostriatal dopamine deficits. Neurobiol. Dis. 2006;21:568–575. - PubMed
-
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J. Cell Sci. 2003;116:217–224. - PubMed
-
- Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J. Biol. Chem. 1983;258:7155–7160. - PubMed
-
- Bonyadi M, Rusholme SA, Cousins FM, Su HC, Biron CA, Farrall M, Akhurst RJ. Mapping of a major genetic modifier of embryonic lethality in TGF beta 1 knockout mice. Nat. Genet. 1997;15:207–211. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

