Myometrial interstitial cells and the coordination of myometrial contractility
- PMID: 19732238
- PMCID: PMC4496132
- DOI: 10.1111/j.1582-4934.2009.00894.x
Myometrial interstitial cells and the coordination of myometrial contractility
Abstract
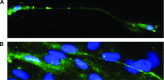
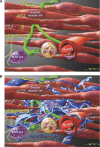
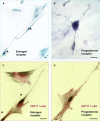
A strict regulation of contractility in the uterus and fallopian tube is essential for various reproductive functions. The uterus contributes, through either increased contractility or periods of relative quiescence, to: (i) expulsion of menstrual debris, (ii) sperm transport, (iii) adequate embryo placement during implantation, (iv) enlarging its capacity during pregnancy and (v) parturition. The dominant cell population of the uterine wall consists of smooth muscle cells that contain the contractile apparatus responsible for the generation of contractile force. Recent interest has focused on a new population of cells located throughout the myometrium on the borders of smooth muscle bundles. These cells are similar to interstitial cells of Cajal (ICC) in the gut that are responsible for the generation of electrical slow waves that control peristalsis. A precise role for myometrial Cajal-like interstitial cells (m-ICLC) has not been identified. m-ICLC express the c-kit receptor, involved in creating and maintaining the ICC phenotype in the gastrointestinal tract. However, both acute and prolonged inhibition of this receptor with the c-kit antagonist imatinib mesylate does not appear to affect the spontaneous contractility of myometrium. Calcium imaging of live tissue slices suggests that contractile signalling starts on the borders of smooth muscle bundles where m-ICLC are located and recently the possible role of extracellular ATP signalling from m-ICLC has been studied. This manuscript reviews the evidence regarding tissue-level signalling in the myometrium with a particular emphasis on the anatomical and possible functional aspects of m-ICLC as new elements of the contractile mechanisms in the uterus.
Figures





Similar articles
-
Uterine contractions depend on KIT-positive interstitial cells in the mouse: genetic and pharmacological evidence.Biol Reprod. 2008 Sep;79(3):510-7. doi: 10.1095/biolreprod.107.066373. Epub 2008 May 14. Biol Reprod. 2008. PMID: 18480468
-
C-kit receptor immunopositive interstitial cells (Cajal-type) in the porcine reproductive tract.Acta Vet Scand. 2017 May 19;59(1):32. doi: 10.1186/s13028-017-0300-5. Acta Vet Scand. 2017. PMID: 28526042 Free PMC article.
-
Immunolocalization and expression of small-conductance calcium-activated potassium channels in human myometrium.J Cell Mol Med. 2012 Dec;16(12):3001-8. doi: 10.1111/j.1582-4934.2012.01627.x. J Cell Mol Med. 2012. PMID: 22947283 Free PMC article.
-
Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour.Eur J Obstet Gynecol Reprod Biol. 2009 May;144 Suppl 1:S2-10. doi: 10.1016/j.ejogrb.2009.02.044. Epub 2009 Mar 18. Eur J Obstet Gynecol Reprod Biol. 2009. PMID: 19299064 Review.
-
Myocytes, myometrium, and uterine contractions.Ann N Y Acad Sci. 2007 Apr;1101:72-84. doi: 10.1196/annals.1389.038. Epub 2007 Apr 18. Ann N Y Acad Sci. 2007. PMID: 17442780 Review.
Cited by
-
Propagation of spontaneous electrical activity in the ex vivo human uterus.Pflugers Arch. 2020 Aug;472(8):1065-1078. doi: 10.1007/s00424-020-02426-w. Epub 2020 Jul 20. Pflugers Arch. 2020. PMID: 32691139 Free PMC article.
-
The Physiology of Reproduction - Quo vadis?Front Physiol. 2021 Mar 30;12:650550. doi: 10.3389/fphys.2021.650550. eCollection 2021. Front Physiol. 2021. PMID: 33859571 Free PMC article. Review.
-
Fish telocytes and their relation to rodlet cells in ruby-red-fin shark (rainbow shark) Epalzeorhynchos frenatum (Teleostei: Cyprinidae).Sci Rep. 2020 Nov 3;10(1):18907. doi: 10.1038/s41598-020-75677-3. Sci Rep. 2020. PMID: 33144597 Free PMC article.
-
Morphological changes in intraepithelial and stromal telocytes in Cyprinus carpio in response to salinity stress.Sci Rep. 2023 Nov 15;13(1):19987. doi: 10.1038/s41598-023-43279-4. Sci Rep. 2023. PMID: 37968439 Free PMC article.
-
TELOCYTES - a case of serendipity: the winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to TELOCYTES.J Cell Mol Med. 2010 Apr;14(4):729-40. doi: 10.1111/j.1582-4934.2010.01059.x. Epub 2010 Mar 26. J Cell Mol Med. 2010. PMID: 20367664 Free PMC article.
References
-
- Gevaert T. Acta biomedical Lovaniensia. Leuven University Press; 2007. Autonomous contractile activity in bladder as a new concept in neuro-urology: a study in rat and mouse Thesis 394,
-
- Drake MJ, Fry CH, Eyden B. Structural characterization of myofibroblasts in the bladder. BJU Int. 2006;97:29–32. - PubMed
-
- Vanderwinden JM, Rumessen JJ, De Laet MH, et al. CD34+ cells in human intestine are fibroblasts adjacent to, but distinct from, interstitial cells of Cajal. Lab Invest. 1999;79:59–65. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

