Antidepressant study design affects patient expectancy: a pilot study
- PMID: 19732481
- PMCID: PMC3784014
- DOI: 10.1017/S0033291709991085
Antidepressant study design affects patient expectancy: a pilot study
Abstract
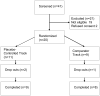
Background: Response to antidepressant medication is higher in comparator versus placebo-controlled randomized controlled trials (RCTs). Patient expectancy is an important influence on clinical outcome in the treatment of depression and may explain this finding. The results are reported from a pilot RCT studying expectancy and depression outcome in placebo-controlled versus comparator treatment conditions.MethodOut-patients aged 18-65 years with major depressive disorder (MDD) were enrolled in this 8-week RCT. Subjects were randomized to placebo-controlled (escitalopram or placebo) or comparator (escitalopram or citalopram) administration of antidepressant medication. Subjects reported their expected likelihood and magnitude of depression improvement before and after randomization using questions from the Credibility and Expectancy Scale (CES). A regressed change model of post-randomization expectancy of improvement was fit to the data to determine whether subjects in the comparator group reported greater expectancies of improvement than subjects in the placebo-controlled group.
Results: Twenty subjects with mean age 56.5+/-11.7 years, a baseline Hamilton Depression Rating Scale (HAMD) score of 24.2+/-5.3, baseline Beck Depression Inventory (BDI) score of 24.9+/-6.4 and baseline Clinical Global Impressions (CGI) - Severity score of 4.0+/-0.3 were enrolled in the study. Adjusting for other factors, the effect of group assignment on expected magnitude of improvement was significant and large (effect size 1.5). No group differences in expected likelihood of improvement were found.
Conclusions: Randomization to comparator versus placebo-controlled administration of antidepressant medication produced greater expectancies of how much patients would improve during the trial. This expectancy difference may explain the higher response and remission rates that are observed in comparator versus placebo-controlled trials.
Conflict of interest statement
The authors report no conflicts of interest relevant to this manuscript.
Figures
References
-
- Beck AT, Ward CH, Mendelson M. An inventory of measuring depression. Archives of General Psychiatry. 1961;4:53–63. - PubMed
-
- Benedetti F, Maggi G, Lopiano L, Lanotte M, Rainero I, Vighetti S, Pollo A. Open versus Hidden Medical Treatments: The Patient's Knowledge about a Therapy Affects the Therapy Outcome. Prevention and Treatment. 2003;6 Available: http://journals.apa.org/journals/pre/6/1/1a.html.
-
- Borkovec TD, Nau SD. Credibility of Analogue Therapy Rationales. Journal of Behavioral Therapy and Experimental Psychiatry. 1972;3:257–260.
-
- Borkovec TD, Costello E. Efficacy of Applied Relaxation and Cognitive-Behavioral Therapy in the Treatment of Generalized Anxiety Disorder. Journal of Consulting and Clinical Psychology. 1993;61:611–619. - PubMed
-
- Cohen J, Cohen P, West SG, Aiken LS. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. 3rd. New York: Lawrence Erlbaum Associates; 2003.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical