Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN
- PMID: 19732719
- PMCID: PMC2754404
- DOI: 10.1016/j.ccr.2009.06.017
Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN
Abstract
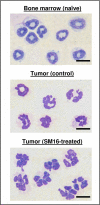
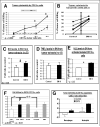
TGF-beta blockade significantly slows tumor growth through many mechanisms, including activation of CD8(+) T cells and macrophages. Here, we show that TGF-beta blockade also increases neutrophil-attracting chemokines, resulting in an influx of CD11b(+)/Ly6G(+) tumor-associated neutrophils (TANs) that are hypersegmented, more cytotoxic to tumor cells, and express higher levels of proinflammatory cytokines. Accordingly, following TGF-beta blockade, depletion of these neutrophils significantly blunts antitumor effects of treatment and reduces CD8(+) T cell activation. In contrast, in control tumors, neutrophil depletion decreases tumor growth and results in more activated CD8(+) T cells intratumorally. Together, these data suggest that TGF-beta within the tumor microenvironment induces a population of TAN with a protumor phenotype. TGF-beta blockade results in the recruitment and activation of TANs with an antitumor phenotype.
Figures





Comment in
-
The yin-yang of tumor-associated neutrophils.Cancer Cell. 2009 Sep 8;16(3):173-4. doi: 10.1016/j.ccr.2009.08.014. Cancer Cell. 2009. PMID: 19732714
References
-
- Allavena P, Sica A, Garlanda C, Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunological Reviews. 2008;222:155–161. - PubMed
-
- Allen SS, Mackie JT, Russell K, Jeevan A, Skwor TA, McMurray DN. Altered inflammatory responses following transforming growth factor-[beta] neutralization in experimental guinea pig tuberculous pleurisy. Tuberculosis. 2008;88:430–436. - PubMed
-
- Appelberg R. Mycobacterial infection primes T cells and macrophages for enhanced recruitment of neutrophils. J Leukoc Biol. 1992;51:472–477. - PubMed
-
- Aruga A, Aruga E, Cameron MJ, Chang AE. Different cytokine profiles released by CD4+ and CD8+ tumor-draining lymph node cells involved in mediating tumor regression. J Leukoc Biol. 1997;61:507–516. - PubMed
-
- Balkwill F, Coussens LM. Cancer: An inflammatory link. Nature. 2004;431:405–406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

