Mutations in LOXHD1, an evolutionarily conserved stereociliary protein, disrupt hair cell function in mice and cause progressive hearing loss in humans
- PMID: 19732867
- PMCID: PMC2771534
- DOI: 10.1016/j.ajhg.2009.07.017
Mutations in LOXHD1, an evolutionarily conserved stereociliary protein, disrupt hair cell function in mice and cause progressive hearing loss in humans
Abstract
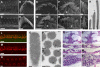
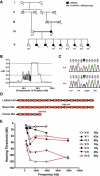
Hearing loss is the most common form of sensory impairment in humans and is frequently progressive in nature. Here we link a previously uncharacterized gene to hearing impairment in mice and humans. We show that hearing loss in the ethylnitrosourea (ENU)-induced samba mouse line is caused by a mutation in Loxhd1. LOXHD1 consists entirely of PLAT (polycystin/lipoxygenase/alpha-toxin) domains and is expressed along the membrane of mature hair cell stereocilia. Stereociliary development is unaffected in samba mice, but hair cell function is perturbed and hair cells eventually degenerate. Based on the studies in mice, we screened DNA from human families segregating deafness and identified a mutation in LOXHD1, which causes DFNB77, a progressive form of autosomal-recessive nonsyndromic hearing loss (ARNSHL). LOXHD1, MYO3a, and PJVK are the only human genes to date linked to progressive ARNSHL. These three genes are required for hair cell function, suggesting that age-dependent hair cell failure is a common mechanism for progressive ARNSHL.
Figures




References
-
- Hildebrand M.S., Newton S.S., Gubbels S.P., Sheffield A.M., Kochhar A., de Silva M.G., Dahl H.H., Rose S.D., Behlke M.A., Smith R.J. Advances in molecular and cellular therapies for hearing loss. Mol. Ther. 2008;16:224–236. - PubMed
-
- Eyken V., Van Camp G., Van Laer L. The complexity of age-related hearing impairment: contributing environmental and genetic factors. Audiol. Neurootol. 2007;12:345–358. - PubMed
-
- Friedman L.M., Dror A.A., Avraham K.B. Mouse models to study inner ear development and hereditary hearing loss. Int. J. Dev. Biol. 2007;51:609–631. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

