Filaggrin deficiency confers a paracellular barrier abnormality that reduces inflammatory thresholds to irritants and haptens
- PMID: 19733297
- PMCID: PMC2881668
- DOI: 10.1016/j.jaci.2009.06.046
Filaggrin deficiency confers a paracellular barrier abnormality that reduces inflammatory thresholds to irritants and haptens
Abstract
Background: Mutations in the human filaggrin gene (FLG) are associated with atopic dermatitis (AD) and are presumed to provoke a barrier abnormality. Yet additional acquired stressors might be necessary because the same mutations can result in a noninflammatory disorder, ichthyosis vulgaris.
Objective: We examined here whether FLG deficiency alone suffices to produce a barrier abnormality, the basis for the putative abnormality, and its proinflammatory consequences.
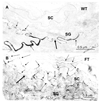
Methods: By using the flaky-tail mouse, which lacks processed murine filaggrin because of a frameshift mutation in the gene encoding profilaggrin that mimics some mutations in human AD, we assessed whether FLG deficiency provokes a barrier abnormality, further localized the defect, identified its subcellular basis, and assessed thresholds to irritant- and hapten-induced dermatitis.
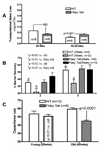
Results: Flaky-tail mice exhibit low-grade inflammation with increased bidirectional, paracellular permeability of water-soluble xenobiotes caused by impaired lamellar body secretion and altered stratum corneum extracellular membranes. This barrier abnormality correlates with reduced inflammatory thresholds to both topical irritants and haptens. Moreover, when exposed repeatedly to topical haptens at doses that produce no inflammation in wild-type mice, flaky-tail mice experience a severe AD-like dermatosis with a further deterioration in barrier function and features of a T(H)2 immunophenotype (increased CRTH levels plus inflammation, increased serum IgE levels, and reduced antimicrobial peptide [mBD3] expression).
Conclusions: FLG deficiency alone provokes a paracellular barrier abnormality in mice that reduces inflammatory thresholds to topical irritants/haptens, likely accounting for enhanced antigen penetration in FLG-associated AD.
Figures





References
-
- Cork MJ, Robinson DA, Vasilopoulos Y, Ferguson A, Moustafa M, MacGowan A, et al. New perspectives on epidermal barrier dysfunction in atopic dermatitis: gene-environment interactions. J Allergy Clin Immunol. 2006;118:3–21. quiz 2–3. - PubMed
-
- Nemoto-Hasebe I, Akiyama M, Nomura T, Sandilands A, McLean WH, Shimizu H. Clinical severity correlates with impaired barrier in filaggrin-related eczema. J Invest Dermatol. 2009;129:682–689. - PubMed
-
- Boguniewicz M, Leung DY. 10. Atopic dermatitis. J Allergy Clin Immunol. 2006;117:S475–S480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

