Autocrine and paracrine angiopoietin 1/Tie-2 signaling promotes muscle satellite cell self-renewal
- PMID: 19733541
- PMCID: PMC4592285
- DOI: 10.1016/j.stem.2009.06.001
Autocrine and paracrine angiopoietin 1/Tie-2 signaling promotes muscle satellite cell self-renewal
Abstract
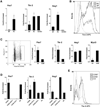
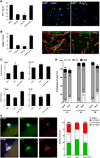
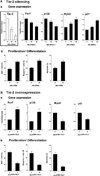
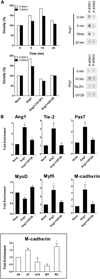
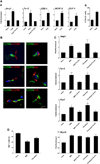
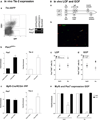
Mechanisms governing muscle satellite cell withdrawal from cell cycle to enter into quiescence remain poorly understood. We studied the role of angiopoietin 1 (Ang1) and its receptor Tie-2 in the regulation of myogenic precursor cell (mpc) fate. In human and mouse, Tie-2 was preferentially expressed by quiescent satellite cells in vivo and reserve cells (RCs) in vitro. Ang1/Tie-2 signaling, through ERK1/2 pathway, decreased mpc proliferation and differentiation, increased the number of cells in G0, increased expression of RC-associated markers (p130, Pax7, Myf-5, M-cadherin), and downregulated expression of differentiation-associated markers. Silencing Tie-2 had opposite effects. Cells located in the satellite cell neighborhood (smooth muscle cells, fibroblasts) upregulated RC-associated markers by secreting Ang1 in vitro. In vivo, Tie-2 blockade and Ang1 overexpression increased the number of cycling and quiescent satellite cells, respectively. We propose that Ang1/Tie-2 signaling regulates mpc self-renewal by controlling the return to quiescence of a subset of satellite cells.
Figures

Comment in
-
Skeletal muscle sings a choral stem cell lullaby.Cell Stem Cell. 2009 Sep 4;5(3):231-2. doi: 10.1016/j.stem.2009.08.012. Cell Stem Cell. 2009. PMID: 19733529
References
-
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. - PubMed
-
- Black EJ, Clark W, Gillespie DA. Transient deactivation of ERK signalling is sufficient for stable entry into G0 in primary avian fibroblasts. Curr. Biol. 2000;10:1119–1122. - PubMed
-
- Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A temporal switch from notch to wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008;2:50–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

