Flow cytometric detection and serotyping of enterovirus for the clinical laboratory
- PMID: 19733594
- PMCID: PMC7172270
- DOI: 10.1016/j.jviromet.2009.08.018
Flow cytometric detection and serotyping of enterovirus for the clinical laboratory
Abstract
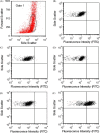
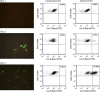
Culture and serotyping of human enteroviruses by fluorescence microscopy are time-consuming and labor-intensive. Flow cytometry has the potential of being more rapid, sensitive, and objective but has not been used for these purposes in a clinical laboratory. Primary rhesus monkey kidney (PMK) cells were inoculated with several enterovirus serotypes and stained with enterovirus-specific antibodies for flow cytometry and indirect fluorescence antibody testing (IFA). Kinetic studies of coxsackievirus B1 and echovirus 30 infection of PMK cells were performed on days 1-4 after inoculation. Flow cytometry results for echovirus 6, 9, 11, and 30 and coxsackievirus B1 correlated with IFA in all cases. Coxsackievirus B1 and echovirus 30 infections were detected 1 day earlier by flow cytometry than IFA. Flow cytometry can be effectively used for detecting enterovirus-infected cells in a clinical laboratory with the advantages of better quantitation of low levels of infection and earlier detection of virally infected cells in culture systems.
Figures

References
-
- Bourlet T., Gharbi J., Omar S., Aouni M., Pozzetto B. Comparison of a rapid culture method combining an immunoperoxidase test and a group specific anti-VP1 monoclonal antibody with conventional virus isolation techniques for routine detection of enteroviruses in stools. J. Med. Virol. 1998;54:204–209. - PubMed
-
- Daley J.K., Gechman L.A., Skipworth J., Rall G.F. Poliovirus replication and spread in primary neuron cultures. Virology. 2005;340:10–20. - PubMed
-
- Freistadt M.S., Eberle K.E. Fluorescent poliovirus for flow cytometric cell surface binding studies. J. Virol. Methods. 2006;134:1–7. - PubMed
-
- Grogan W.L., Collins J.M. Marcel Dekker; New York: 1990. Guide to Flow Cytometry Methods.
-
- Khetsuriani N., Lamonte-Fowlkes A., Oberst S., Pallansch M.A. Enterovirus surveillance—United States, 1970–2005. MMWR Surveill. Summ. 2006;55:1–20. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

