Spemann's organizer and the self-regulation of embryonic fields
- PMID: 19733655
- PMCID: PMC2803698
- DOI: 10.1016/j.mod.2009.08.004
Spemann's organizer and the self-regulation of embryonic fields
Abstract
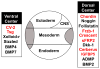
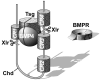
Embryos and developing organs have the remarkable ability of self-regenerating after experimental manipulations. In the Xenopus blastula half-embryos can regenerate the missing part, producing identical twins. Studies on the molecular nature of Spemann's organizer have revealed that self-regulation results from the battle between two signaling centers under reciprocal transcriptional control. Long-range communication between the dorsal and ventral sides is mediated by the action of growth factor antagonists - such as the BMP antagonist Chordin - that regulate the flow of BMPs within the embryonic morphogenetic field. BMPs secreted by the dorsal Spemann organizer tissue are released by metalloproteinases of the Tolloid family, which cleave Chordin at a distance of where they were produced. The dorsal center secretes Chordin, Noggin, BMP2 and ADMP. The ventral center of the embryo secretes BMP4, BMP7, Sizzled, Crossveinless-2 and Tolloid-related. Crossveinless-2 binds Chordin/BMP complexes, facilitating their flow towards the ventral side, where BMPs are released by Tolloid allowing peak BMP signaling. Self-regulation occurs because transcription of ventral genes is induced by BMP while transcription of dorsal genes is repressed by BMP signals. This assures that for each action of Spemann's organizer there is a reaction in the ventral side of the embryo. Because both dorsal and ventral centers express proteins of similar biochemical activities, they can compensate for each other. A novel biochemical pathway of extracellular growth factor signaling regulation has emerged from these studies in Xenopus. This remarkable dorsal-ventral positional information network has been conserved in evolution and is ancestral to all bilateral animals.
Figures











References
-
- Akiyama-Oda Y, Oda H. Axis specification in the spider embryo: dpp is required for radial-to-axial symmetry transformation and sog for ventral patterning. Development. 2006;133:2347–2357. - PubMed
-
- Appel TA. The Cuvier-Geoffroy Debate. Oxford University Press; Oxford: 1987.
-
- Bachiller D, et al. The organizer secreted factors Chordin and Noggin are required for forebrain development in the mouse. Nature. 2000;403:658–661. - PubMed
-
- Barth LG. Neural diffferentiation without organizer. J Exp Zool. 1941;87:371–384.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

