The Sts proteins target tyrosine phosphorylated, ubiquitinated proteins within TCR signaling pathways
- PMID: 19733910
- PMCID: PMC2757469
- DOI: 10.1016/j.molimm.2009.08.015
The Sts proteins target tyrosine phosphorylated, ubiquitinated proteins within TCR signaling pathways
Abstract
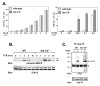
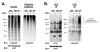
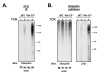
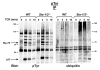
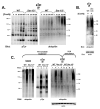
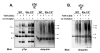
The T cell receptor (TCR) detects the presence of infectious pathogens and activates numerous intracellular signaling pathways. Protein tyrosine phosphorylation and ubiquitination serve as key regulatory mechanisms downstream of the TCR. Negative regulation of TCR signaling pathways is important in controlling the immune response, and the Suppressor of TCR Signaling proteins (Sts-1 and Sts-2) have been shown to function as critical negative regulators of TCR signaling. Although their mechanism of action has yet to be fully uncovered, it is known that the Sts proteins possess intrinsic phosphatase activity. Here, we demonstrate that Sts-1 and Sts-2 are instrumental in down-modulating proteins that are dually modified by both protein tyrosine phosphorylation and ubiquitination. Specifically, both naïve and activated T cells derived from genetically engineered mice that lack the Sts proteins display strikingly elevated levels of tyrosine phosphorylated, ubiquitinated proteins following TCR stimulation. The accumulation of the dually modified proteins is transient, and in activated T cells but not naïve T cells is significantly enhanced by co-receptor engagement. Our observations hint at a novel regulatory mechanism downstream of the T cell receptor.
Figures
References
-
- Acuto O, Bartolo VD, Michel F. Tailoring T-cell receptor signals by proximal negative feedback mechanisms. Nat. Rev. Immunol. 2008;8:699–712. - PubMed
-
- Agrawal R, Carpino N, Tsygankov A. TULA proteins regulate activity of the protein tyrosine kinase Syk. J. Cell. Biochem. 2008;104:953–964. - PubMed
-
- Carpino N, Turner S, Mekala D, Takahashi Y, Zang H, Geiger TL, Doherty P, Ihle JN. Regulation of Zap-70 activation and TCR signaling by two related proteins, Sts-1 and Sts-2. Immunity. 2004;20:37–46. - PubMed
-
- Cenciarelli C, Hou D, Hsu KC, Rellahan BL, Wiest DL, Smith HT, Fried VA, Weissman AM. Activation-induced ubiquitination of the T cell antigen receptor. Science. 1992;257:795–797. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

