Coordination of polymerization, chain termination, and export in assembly of the Escherichia coli lipopolysaccharide O9a antigen in an ATP-binding cassette transporter-dependent pathway
- PMID: 19734145
- PMCID: PMC2781620
- DOI: 10.1074/jbc.M109.052878
Coordination of polymerization, chain termination, and export in assembly of the Escherichia coli lipopolysaccharide O9a antigen in an ATP-binding cassette transporter-dependent pathway
Abstract
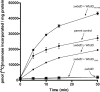
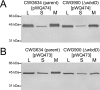
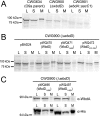
The Escherichia coli O9a O-polysaccharide (O-PS) is a prototype for O-PS synthesis and export by the ATP-binding cassette transporter-dependent pathway. Comparable systems are widespread in Gram-negative bacteria. The polymannose O9a O-PS is assembled on a polyisoprenoid lipid intermediate by mannosyltransferases located at the cytoplasmic membrane, and the final polysaccharide chain length is determined by the chain terminating dual kinase/methyltransferase, WbdD. The WbdD protein is tethered to the membrane via a C-terminal region containing amphipathic helices located between residues 601 and 669. Here, we establish that the C-terminal domain of WbdD plays an additional pivotal role in assembly of the O-PS by forming a complex with the chain-extending mannosyltransferase, WbdA. Membrane preparations from a DeltawbdD mutant had severely diminished mannosyltransferase activity in vitro, and no significant amounts of the WbdA protein are targeted to the membrane fraction. Expression of a polypeptide comprising the WbdD C-terminal region was sufficient to restore both proper localization of WbdA and mannosyltransferase activity. In contrast to WbdA, the other required mannosyltransferases (WbdBC) are targeted to the membrane independent of WbdD. A bacterial two-hybrid system confirmed the interaction of WbdD and WbdA and identified two regions in the C terminus of WbdD that contributed to the interaction. Therefore, in the O9a assembly export system, the WbdD protein orchestrates the critical localization and coordination of activities involved in O-PS chain extension and termination at the cytoplasmic membrane.
Figures


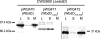

Similar articles
-
In vitro reconstruction of the chain termination reaction in biosynthesis of the Escherichia coli O9a O-polysaccharide: the chain-length regulator, WbdD, catalyzes the addition of methyl phosphate to the non-reducing terminus of the growing glycan.J Biol Chem. 2011 Dec 2;286(48):41391-41401. doi: 10.1074/jbc.M111.295857. Epub 2011 Oct 11. J Biol Chem. 2011. PMID: 21990359 Free PMC article.
-
Domain interactions control complex formation and polymerase specificity in the biosynthesis of the Escherichia coli O9a antigen.J Biol Chem. 2015 Jan 9;290(2):1075-85. doi: 10.1074/jbc.M114.622480. Epub 2014 Nov 24. J Biol Chem. 2015. PMID: 25422321 Free PMC article.
-
Structure of WbdD: a bifunctional kinase and methyltransferase that regulates the chain length of the O antigen in Escherichia coli O9a.Mol Microbiol. 2012 Nov;86(3):730-42. doi: 10.1111/mmi.12014. Epub 2012 Sep 27. Mol Microbiol. 2012. PMID: 22970759 Free PMC article.
-
Synthesis of lipopolysaccharide O-antigens by ABC transporter-dependent pathways.Carbohydr Res. 2012 Jul 15;356:12-24. doi: 10.1016/j.carres.2012.02.027. Epub 2012 Mar 6. Carbohydr Res. 2012. PMID: 22475157 Review.
-
New insights into lipopolysaccharide assembly and export.Curr Opin Chem Biol. 2019 Dec;53:37-43. doi: 10.1016/j.cbpa.2019.07.004. Epub 2019 Aug 21. Curr Opin Chem Biol. 2019. PMID: 31445442 Review.
Cited by
-
In vitro reconstruction of the chain termination reaction in biosynthesis of the Escherichia coli O9a O-polysaccharide: the chain-length regulator, WbdD, catalyzes the addition of methyl phosphate to the non-reducing terminus of the growing glycan.J Biol Chem. 2011 Dec 2;286(48):41391-41401. doi: 10.1074/jbc.M111.295857. Epub 2011 Oct 11. J Biol Chem. 2011. PMID: 21990359 Free PMC article.
-
Discovery of monoclonal antibodies cross-reactive to novel subserotypes of K. pneumoniae O3.Sci Rep. 2017 Jul 26;7(1):6635. doi: 10.1038/s41598-017-06682-2. Sci Rep. 2017. PMID: 28747785 Free PMC article.
-
Antigenic Variation among Bordetella: Bordetella bronchiseptica strain MO149 expresses a novel o chain that is poorly immunogenic.J Biol Chem. 2010 Aug 27;285(35):26869-26877. doi: 10.1074/jbc.M110.115121. Epub 2010 Jun 30. J Biol Chem. 2010. PMID: 20592026 Free PMC article.
-
Five new genes are important for common polysaccharide antigen biosynthesis in Pseudomonas aeruginosa.mBio. 2013 Jan 22;4(1):e00631-12. doi: 10.1128/mBio.00631-12. mBio. 2013. PMID: 23341552 Free PMC article.
-
Crystallization, dehydration and experimental phasing of WbdD, a bifunctional kinase and methyltransferase from Escherichia coli O9a.Acta Crystallogr D Biol Crystallogr. 2012 Oct;68(Pt 10):1371-9. doi: 10.1107/S0907444912029599. Epub 2012 Sep 18. Acta Crystallogr D Biol Crystallogr. 2012. PMID: 22993091 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

