The intestine-specific transcription factor Cdx2 inhibits beta-catenin/TCF transcriptional activity by disrupting the beta-catenin-TCF protein complex
- PMID: 19734199
- PMCID: PMC2812568
- DOI: 10.1093/carcin/bgp213
The intestine-specific transcription factor Cdx2 inhibits beta-catenin/TCF transcriptional activity by disrupting the beta-catenin-TCF protein complex
Abstract
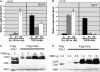
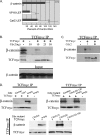
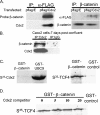
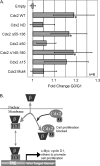
Cdx2 is an intestine-specific transcription factor known to regulate proliferation and differentiation. We have reported previously that Cdx2 limits the proliferation of human colon cancer cells by inhibiting the transcriptional activity of the beta-catenin-T-cell factor (TCF) bipartite complex. Herein we further elucidate this mechanism. Studies with a classic Cdx2 target gene and a canonical Wnt/beta-catenin/TCF reporter suggest that Cdx2 regulates these promoters by distinctly different processes. Specifically, inhibition of beta-catenin/TCF activity by Cdx2 does not require Cdx2 transcriptional activity. Instead, Cdx2 binds beta-catenin and disrupts its interaction with the DNA-binding TCF factors, thereby silencing beta-catenin/TCF target gene expression. Using Cdx2 mutants, we map the Cdx2 domains required for the inhibition of beta-catenin/TCF activity. We identify a subdomain in the N-terminus that is highly conserved and when mutated significantly reduces Cdx2 inhibition of beta-catenin/TCF transcriptional activity. Mutation of this subdomain also abrogates Cdx2's anti-proliferative effects in colon cancer cells. In summary, we conclude that Cdx2 binds beta-catenin and disrupts the beta-catenin-TCF complex. Considering the pivotal role of beta-catenin/TCF activity in driving proliferation of normal intestinal epithelial and colon cancer cells, our findings suggest a novel mechanism for Cdx2-mediated regulation of Wnt/beta-catenin signaling and cell proliferation.
Figures


Similar articles
-
Cdx1 inhibits human colon cancer cell proliferation by reducing beta-catenin/T-cell factor transcriptional activity.J Biol Chem. 2004 Aug 27;279(35):36865-75. doi: 10.1074/jbc.M405213200. Epub 2004 Jun 23. J Biol Chem. 2004. PMID: 15215241
-
Sox17 and Sox4 differentially regulate beta-catenin/T-cell factor activity and proliferation of colon carcinoma cells.Mol Cell Biol. 2007 Nov;27(22):7802-15. doi: 10.1128/MCB.02179-06. Epub 2007 Sep 17. Mol Cell Biol. 2007. PMID: 17875931 Free PMC article.
-
Pinin modulates expression of an intestinal homeobox gene, Cdx2, and plays an essential role for small intestinal morphogenesis.Dev Biol. 2010 Sep 15;345(2):191-203. doi: 10.1016/j.ydbio.2010.07.009. Epub 2010 Jul 14. Dev Biol. 2010. PMID: 20637749 Free PMC article.
-
The Yin-Yang of TCF/beta-catenin signaling.Adv Cancer Res. 2000;77:1-24. doi: 10.1016/s0065-230x(08)60783-6. Adv Cancer Res. 2000. PMID: 10549354 Review.
-
Interaction of nuclear receptors with the Wnt/beta-catenin/Tcf signaling axis: Wnt you like to know?Endocr Rev. 2005 Dec;26(7):898-915. doi: 10.1210/er.2003-0034. Epub 2005 Aug 26. Endocr Rev. 2005. PMID: 16126938 Review.
Cited by
-
Caudal homeobox protein Cdx-2 cooperates with Wnt pathway to regulate claudin-1 expression in colon cancer cells.PLoS One. 2012;7(6):e37174. doi: 10.1371/journal.pone.0037174. Epub 2012 Jun 15. PLoS One. 2012. PMID: 22719836 Free PMC article.
-
Ectopic expression of Ptf1a induces spinal defects, urogenital defects, and anorectal malformations in Danforth's short tail mice.PLoS Genet. 2013;9(2):e1003204. doi: 10.1371/journal.pgen.1003204. Epub 2013 Feb 21. PLoS Genet. 2013. PMID: 23436999 Free PMC article.
-
Wnt signaling specifies and patterns intestinal endoderm.Mech Dev. 2011 Sep-Dec;128(7-10):387-400. doi: 10.1016/j.mod.2011.07.005. Epub 2011 Aug 10. Mech Dev. 2011. PMID: 21854845 Free PMC article.
-
Mitochondrial translocator protein deficiency exacerbates pathology in acute experimental ulcerative colitis.Front Physiol. 2022 Aug 19;13:896951. doi: 10.3389/fphys.2022.896951. eCollection 2022. Front Physiol. 2022. PMID: 36060674 Free PMC article.
-
Recombinant lentivirus with enhanced expression of caudal-related homeobox protein 2 inhibits human colorectal cancer cell proliferation in vitro.Mol Med Rep. 2015 Aug;12(2):1838-44. doi: 10.3892/mmr.2015.3594. Epub 2015 Apr 3. Mol Med Rep. 2015. PMID: 25847407 Free PMC article.
References
-
- Lynch JP, et al. The genetic pathogenesis of colorectal cancer. Hematol. Oncol. Clin. North Am. 2002;16:1–36. - PubMed
-
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. - PubMed
-
- Korinek V, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 1998;19:379–383. - PubMed
-
- Guo RJ, et al. The role of Cdx proteins in intestinal development and cancer. Cancer Biol. Ther. 2004;3:593–601. - PubMed

