The cyclization of the 3,6-anhydro-galactose ring of iota-carrageenan is catalyzed by two D-galactose-2,6-sulfurylases in the red alga Chondrus crispus
- PMID: 19734263
- PMCID: PMC2773109
- DOI: 10.1104/pp.109.144329
The cyclization of the 3,6-anhydro-galactose ring of iota-carrageenan is catalyzed by two D-galactose-2,6-sulfurylases in the red alga Chondrus crispus
Abstract
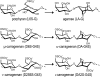
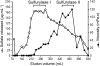
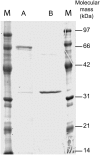
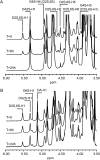
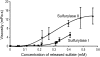
Carrageenans are sulfated galactans found in the cell walls of numerous red seaweeds (Rhodophyta). They are classified according to the number and the position of sulfate ester groups and the occurrence of 3,6-anhydro-galactose. Although the carrageenan biosynthesis pathway is not fully understood, it is usually accepted that the last step consists of the formation of a 3,6-anhydro ring found in kappa- and iota-carrageenans through the enzymatic conversion of d-galactose-6-sulfate or d-galactose-2,6-disulfate occurring in mu- and nu-carrageenan, respectively. We purified two enzymes, sulfurylase I (65 kD) and sulfurylase II (32 kD), that are able to catalyze the conversion of nu- into iota-carrageenan. We compared their sulfate release rates (i.e. arising from the formation of the anhydro ring) with the viscosity of the solution and demonstrated two distinct modes of action. In addition, we found that some mixtures of sulfurylase I and II lead to the formation of carrageenan solutions with unexpectedly low viscosities. We discuss the implication of these findings for the assembly of a densely aggregated matrix in red algal cell walls.
Figures

References
-
- Bellion C, Brigand G, Prome JC, Welti D, Bociek S (1983) Identification et caractérisation des précurseurs biologiques des carraghénanes par spectroscopie de R.M.N.-13C. Carbohydr Res 119 31–48
-
- Bixler H (1996) Recent developments in manufacturing and marketing carrageenan. Hydrobiologia 326/327 35–37
-
- Bixler H, Johndro K, Falshaw R (2001) Kappa-2 carrageenan: structure and performance of commercial extracts. II. Performance in two simulated dairy applications. Food Hydrocoll 15 619–630
-
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248–254 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

