Opening a new path to lipoic acid
- PMID: 19734307
- PMCID: PMC2772479
- DOI: 10.1128/JB.01151-09
Opening a new path to lipoic acid
Figures
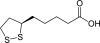
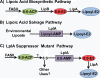
Comment on
-
Scavenging of cytosolic octanoic acid by mutant LplA lipoate ligases allows growth of Escherichia coli strains lacking the LipB octanoyltransferase of lipoic acid synthesis.J Bacteriol. 2009 Nov;191(22):6796-803. doi: 10.1128/JB.00798-09. Epub 2009 Aug 14. J Bacteriol. 2009. PMID: 19684135 Free PMC article.
References
-
- Cronan, J. E., X. Zhao, and Y. Jiang. 2005. Function, attachment and synthesis of lipoic acid in Escherichia coli. Adv. Microb. Physiol. 50:103-146. - PubMed
-
- Fujiwara, K., S. Toma, K. Okamura-Ikeda, Y. Motokawa, A. Nakagawa, and H. Taniguchi. 2005. Crystal structure of lipoate-protein ligase A from Escherichia coli. Determination of the lipoic acid-binding site. J. Biol. Chem. 280:33645-33651. - PubMed
-
- Herbert, A. A., and J. R. Guest. 1968. Biochemical and genetic studies with lysine+methionine mutants of Escherichia coli: lipoic acid and α-ketoglutarate dehydrogenase-less mutants. J. Gen. Microbiol. 53:363-381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

