Small stress response proteins in Escherichia coli: proteins missed by classical proteomic studies
- PMID: 19734316
- PMCID: PMC2798279
- DOI: 10.1128/JB.00872-09
Small stress response proteins in Escherichia coli: proteins missed by classical proteomic studies
Abstract
Proteins of 50 or fewer amino acids are poorly characterized in all organisms. The corresponding genes are challenging to reliably annotate, and it is difficult to purify and characterize the small protein products. Due to these technical limitations, little is known about the abundance of small proteins, not to mention their biological functions. To begin to characterize these small proteins in Escherichia coli, we assayed their accumulation under a variety of growth conditions and after exposure to stress. We found that many small proteins accumulate under specific growth conditions or are stress induced. For some genes, the observed changes in protein levels were consistent with known transcriptional regulation, such as ArcA activation of the operons encoding yccB and ybgT. However, we also identified novel regulation, such as Zur repression of ykgMO, cyclic AMP response protein (CRP) repression of azuC, and CRP activation of ykgR. The levels of 11 small proteins increase after heat shock, and induction of at least 1 of these, YobF, occurs at a posttranscriptional level. These results show that small proteins are an overlooked subset of stress response proteins in E. coli and provide information that will be valuable for determining the functions of these proteins.
Figures
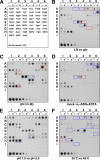



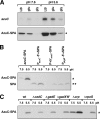

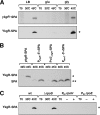

Comment in
-
Global approaches for finding small RNA and small open reading frame functions.J Bacteriol. 2010 Jan;192(1):26-8. doi: 10.1128/JB.01316-09. J Bacteriol. 2010. PMID: 19854892 Free PMC article. No abstract available.
References
-
- Ades, S. E. 2008. Regulation by destruction: design of the σE envelope stress response. Curr. Opin. Microbiol. 11:535-540. - PubMed
-
- Burkholder, W. F., I. Kurtser, and A. D. Grossman. 2001. Replication initiation proteins regulate a developmental checkpoint in Bacillus subtilis. Cell 104:269-279. - PubMed
-
- Castanie-Cornet, M. P., and J. W. Foster. 2001. Escherichia coli acid resistance: cAMP receptor protein and a 20 bp cis-acting sequence control pH and stationary phase expression of the gadA and gadBC glutamate decarboxylase genes. Microbiology 147:709-715. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

