Innate immune response during Yersinia infection: critical modulation of cell death mechanisms through phagocyte activation
- PMID: 19734471
- PMCID: PMC2774879
- DOI: 10.1189/jlb.0309146
Innate immune response during Yersinia infection: critical modulation of cell death mechanisms through phagocyte activation
Abstract
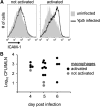
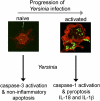
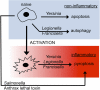
Yersinia pestis, the etiological agent of plague, is one of the most deadly pathogens on our planet. This organism shares important attributes with its ancestral progenitor, Yersinia pseudotuberculosis, including a 70-kb virulence plasmid, lymphotropism during growth in the mammalian host, and killing of host macrophages. Infections with both organisms are biphasic, where bacterial replication occurs initially with little inflammation, followed by phagocyte influx, inflammatory cytokine production, and tissue necrosis. During infection, plasmid-encoded attributes facilitate bacterial-induced macrophage death, which results from two distinct processes and corresponds to the inflammatory crescendo observed in vivo: Naïve cells die by apoptosis (noninflammatory), and later in infection, activated macrophages die by pyroptosis (inflammatory). The significance of this redirected cell death for the host is underscored by the importance of phagocyte activation for immunity to Yersinia and the protective role of pyroptosis during host responses to anthrax lethal toxin and infections with Francisella, Legionella, Pseudomonas, and Salmonella. The similarities of Y. pestis and Y. pseudotuberculosis, including conserved, plasmid-encoded functions inducing at least two distinct mechanisms of cell death, indicate that comparative studies are revealing about their critical pathogenic mechanism(s) and host innate immune responses during infection. Validation of this idea and evidence of similar interactions with the host immune system are provided by Y. pseudotuberculosis-priming, cross-protective immunity against Y. pestis. Despite these insights, additional studies indicate much remains to be understood concerning effective host responses against Yersinia, including chromosomally encoded attributes that also contribute to bacterial evasion and modulation of innate and adaptive immune responses.
Figures
References
-
- Inglesby T V, Dennis D T, Henderson D A, Bartlett J G, Ascher M S, Eitzen E, Fine A D, Friedlander A M, Hauer J, Koerner J F, Layton M, McDade J, Osterholm M T, O'Toole T, Parker G, Perl T M, Russell P K, Schoch-Spana M, Tonat K. Plague as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA. 2000;283:2281–2290. - PubMed
-
- Koirala J. Plague: disease, management, and recognition of act of terrorism. Infect Dis Clin North Am. 2006;20:273–287. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

