A matrix metalloproteinase-1/protease activated receptor-1 signaling axis promotes melanoma invasion and metastasis
- PMID: 19734937
- PMCID: PMC2788659
- DOI: 10.1038/onc.2009.272
A matrix metalloproteinase-1/protease activated receptor-1 signaling axis promotes melanoma invasion and metastasis
Abstract
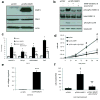
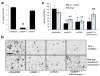
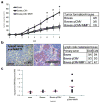
Hallmarks of malignant melanoma are its propensity to metastasize and its resistance to treatment, giving patients with advanced disease a poor prognosis. The transition of melanoma from non-invasive radial growth phase (RGP) to invasive and metastatically competent vertical growth phase (VGP) is a major step in tumor progression, yet the mechanisms governing this transformation are unknown. Matrix metalloproteinase-1 (MMP-1) is highly expressed by VGP melanomas, and is thought to contribute to melanoma progression by degrading type I collagen within the skin to facilitate melanoma invasion. Protease activated receptor-1 (PAR-1) is activated by MMP-1, and is also expressed by VGP melanomas. However, the effects of MMP-1 signaling through PAR-1 have not been examined in melanoma. Here, we demonstrate that an MMP-1/PAR-1 signaling axis exists in VGP melanoma, and is necessary for melanoma invasion. Introduction of MMP-1 into RGP melanoma cells induced gene expression associated with tumor progression and promoted invasion in vitro, and enhanced tumor growth and conferred metastatic capability in vivo. This study demonstrates that both the type I collagenase and PAR-1 activating functions of MMP-1 are required for melanoma progression, and suggests that MMP-1 may be a major contributor to the transformation of melanoma from non-invasive to malignant disease.
Conflict of interest statement
Figures



References
-
- Arora P, Ricks TK, Trejo J. Protease-activated receptor signalling, endocytic sorting and dysregulation in cancer. J Cell Sci. 2007;120:921–928. - PubMed
-
- Balch C, Soong SJ, Atkins M, Buzaid A, Thompson J. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54:131–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

