Analysis of differential expression of glycosyltransferases in healing corneas by glycogene microarrays
- PMID: 19736239
- PMCID: PMC2782180
- DOI: 10.1093/glycob/cwp133
Analysis of differential expression of glycosyltransferases in healing corneas by glycogene microarrays
Erratum in
-
Analysis of differential expression of glycosyltransferases in healing corneas by glycogene microarrays.Glycobiology. 2019 Feb 1;29(2):188-189. doi: 10.1093/glycob/cwy076. Glycobiology. 2019. PMID: 30508096 Free PMC article. No abstract available.
Abstract
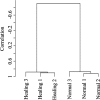
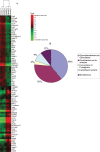
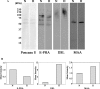
It is generally accepted that the glycans on the cell surface and extracellular matrix proteins play a pivotal role in the events that mediate re-epithelialization of wounds. Yet, the global alteration in the structure and composition of glycans, specifically occurring during corneal wound closure remains unknown. In this study, GLYCOv2 glycogene microarray technology was used for the first time to identify the differentially expressed glycosylation-related genes in healing mouse corneas. Of approximately 2000 glycogenes on the array, the expression of 11 glycosytransferase and glycosidase enzymes was upregulated and that of 19 was downregulated more than 1.5-fold in healing corneas compared with the normal, uninjured corneas. Among them, notably, glycosyltransferases, beta3GalT5, T-synthase, and GnTIVb, were all found to be induced in the corneas in response to injury, whereas, GnTIII and many sialyltransferases were downregulated. Interestingly, it appears that the glycan structures on glycoproteins and glycolipids, expressed in healing corneas as a result of differential regulation of these glycosyltransferases, may serve as specific counter-receptors for galectin-3, a carbohydrate-binding protein, known to play a key role in re-epithelialization of corneal wounds. Additionally, many glycogenes including a proteoglycan, glypican-3, cell adhesion proteins dectin-1 and -2, and mincle, and mucin 1 were identified for the first time to be differentially regulated during corneal wound healing. Results of glycogene microarray data were confirmed by qRT-PCR and lectin blot analyses. The differentially expressed glycogenes identified in the present study have not previously been investigated in the context of wound healing and represent novel factors for investigating the role of carbohydrate-mediated recognition in corneal wound healing.
Figures

References
-
- Almkvist J, Dahlgren C, Leffler H, Karlsson A. Newcastle disease virus neuraminidase primes neutrophils for stimulation by galectin-3 and formyl-Met-Leu-Phe. Exp Cell Res. 2004;298:74–82. - PubMed
-
- Amado M, Almeida R, Schwientek T, Clausen H. Identification and characterization of large galactosyltransferase gene families: Galactosyltransferases for all functions. Biochim Biophys Acta. 1999;1473:35–53. - PubMed
-
- Amano M, Galvan M, He J, Baum LG. The ST6Gal I sialyltransferase selectively modifies N-glycans on CD45 to negatively regulate galectin-1-induced CD45 clustering, phosphatase modulation, and T cell death. J Biol Chem. 2003;278:7469–7475. - PubMed
-
- Brewer CF. Thermodynamic binding studies of galectin-1, -3 and -7. Glycoconj J. 2004;19:459–465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

