The effects of excluding patients from the analysis in randomised controlled trials: meta-epidemiological study
- PMID: 19736281
- PMCID: PMC2739282
- DOI: 10.1136/bmj.b3244
The effects of excluding patients from the analysis in randomised controlled trials: meta-epidemiological study
Abstract
Objective: To examine whether excluding patients from the analysis of randomised trials are associated with biased estimates of treatment effects and higher heterogeneity between trials.
Design: Meta-epidemiological study based on a collection of meta-analyses of randomised trials.
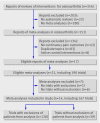
Data sources: 14 meta-analyses including 167 trials that compared therapeutic interventions with placebo or non-intervention control in patients with osteoarthritis of the hip or knee and used patient reported pain as an outcome.
Methods: Effect sizes were calculated from differences in means of pain intensity between groups at the end of follow-up, divided by the pooled standard deviation. Trials were combined by using random effects meta-analysis. Estimates of treatment effects were compared between trials with and trials without exclusions from the analysis, and the impact of restricting meta-analyses to trials without exclusions was assessed.
Results: 39 trials (23%) had included all patients in the analysis. In 128 trials (77%) some patients were excluded from the analysis. Effect sizes from trials with exclusions tended to be more beneficial than those from trials without exclusions (difference -0.13, 95% confidence interval -0.29 to 0.04). However, estimates of bias between individual meta-analyses varied considerably (tau(2)=0.07). Tests of interaction between exclusions from the analysis and estimates of treatment effects were positive in five meta-analyses. Stratified analyses indicated that differences in effect sizes between trials with and trials without exclusions were more pronounced in meta-analyses with high between trial heterogeneity, in meta-analyses with large estimated treatment benefits, and in meta-analyses of complementary medicine. Restriction of meta-analyses to trials without exclusions resulted in smaller estimated treatment benefits, larger P values, and considerable decreases in between trial heterogeneity.
Conclusion: Excluding patients from the analysis in randomised trials often results in biased estimates of treatment effects, but the extent and direction of bias is unpredictable. Results from intention to treat analyses should always be described in reports of randomised trials. In systematic reviews, the influence of exclusions from the analysis on estimated treatment effects should routinely be assessed.
Conflict of interest statement
Competing interests: None declared.
Figures



References
-
- Tierney JF, Stewart LA. Investigating patient exclusion bias in meta-analysis. Int J Epidemiol 2005;34:79-87. - PubMed
-
- Sackett DL, Gent M. Controversy in counting and attributing events in clinical trials. N Engl J Med 1979;301:1410-2. - PubMed
-
- Coronary Drug Project Research Group. Influence of adherence to treatment and response of cholesterol on mortality in the coronary drug project. N Engl J Med 1980;303:1038-41. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
