Loss of sensory input increases the intrinsic excitability of layer 5 pyramidal neurons in rat barrel cortex
- PMID: 19736297
- PMCID: PMC2790252
- DOI: 10.1113/jphysiol.2009.180943
Loss of sensory input increases the intrinsic excitability of layer 5 pyramidal neurons in rat barrel cortex
Abstract
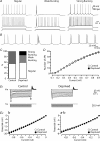
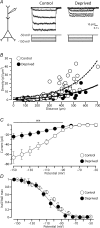
Development of the cortical map is experience dependent, with different critical periods in different cortical layers. Previous work in rodent barrel cortex indicates that sensory deprivation leads to changes in synaptic transmission and plasticity in layer 2/3 and 4. Here, we studied the impact of sensory deprivation on the intrinsic properties of layer 5 pyramidal neurons located in rat barrel cortex using simultaneous somatic and dendritic recording. Sensory deprivation was achieved by clipping all the whiskers on one side of the snout. Loss of sensory input did not change somatic active and resting membrane properties, and did not influence dendritic action potential (AP) backpropagation. In contrast, sensory deprivation led to an increase in the percentage of layer 5 pyramidal neurons showing burst firing. This was associated with a reduction in the threshold for generation of dendritic calcium spikes during high-frequency AP trains. Cell-attached recordings were used to assess changes in the properties and expression of dendritic HCN channels. These experiments indicated that sensory deprivation caused a decrease in HCN channel density in distal regions of the apical dendrite. To assess the contribution of HCN down-regulation on the observed increase in dendritic excitability following sensory deprivation, we investigated the impact of blocking HCN channels. Block of HCN channels removed differences in dendritic calcium electrogenesis between control and deprived neurons. In conclusion, these observations indicate that sensory loss leads to increased dendritic excitability of cortical layer 5 pyramidal neurons. Furthermore, they suggest that increased dendritic calcium electrogenesis following sensory deprivation is mediated in part via down-regulation of dendritic HCN channels.
Figures
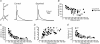


References
-
- Aizenman CD, Akerman CJ, Jensen KR, Cline HT. Visually driven regulation of intrinsic neuronal excitability improves stimulus detection in vivo. Neuron. 2003;39:831–842. - PubMed
-
- Allen CB, Celikel T, Feldman DE. Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat Neurosci. 2003;6:291–299. - PubMed
-
- Armstrong-James M, Callahan CA, Friedman MA. Thalamo-cortical processing of vibrissal information in the rat. I. Intracortical origins of surround but not centre-receptive fields of layer IV neurones in the rat S1 barrel field cortex. J Comp Neurol. 1991;303:193–210. - PubMed
-
- Armstrong-James M, Fox K. Spatiotemporal convergence and divergence in the rat S1 ‘barrel’ cortex. J Comp Neurol. 1987;263:265–281. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

