Myostatin represses physiological hypertrophy of the heart and excitation-contraction coupling
- PMID: 19736304
- PMCID: PMC2770153
- DOI: 10.1113/jphysiol.2009.172544
Myostatin represses physiological hypertrophy of the heart and excitation-contraction coupling
Abstract
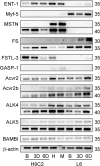
Although myostatin negatively regulates skeletal muscle growth, its function in heart is virtually unknown. Herein we demonstrate that it inhibits basal and IGF-stimulated proliferation and differentiation and also modulates cardiac excitation-contraction (EC) coupling. Loss of myostatin induced eccentric hypertrophy and enhanced cardiac responsiveness to beta-adrenergic stimulation in vivo. This was due to myostatin null ventricular myocytes having larger [Ca(2+)](i) transients and contractions and responding more strongly to beta-adrenergic stimulation than wild-type cells. Enhanced cardiac output and beta-adrenergic responsiveness of myostatin null mice was therefore due to increased SR Ca(2+) release during EC coupling and to physiological hypertrophy, but not to enhanced myofilament function as determined by simultaneous measurement of force and ATPase activity. Our studies support the novel concept that myostatin is a repressor of physiological cardiac muscle growth and function. Thus, the controlled inhibition of myostatin action could potentially help repair damaged cardiac muscle by inducing physiological hypertrophy.
Figures

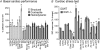

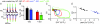
Comment in
-
Take it to heart: myostatin inhibition, mighty mouse and the quest for a competitive edge.J Physiol. 2009 Nov 1;587(Pt 21):5005. doi: 10.1113/jphysiol.2009.181487. J Physiol. 2009. PMID: 19880873 Free PMC article. No abstract available.
References
-
- Abergel E, Chatellier G, Hagege AA, Oblak A, Linhart A, Ducardonnet A, Menard J. Serial left ventricular adaptations in world-class professional cyclists: implications for disease screening and follow-up. J Am Coll Cardiol. 2004;44:144–149. - PubMed
-
- Adams GR. Invited Review: Autocrine/paracrine IGF-I and skeletal muscle adaptation. J Appl Physiol. 2002;93:1159–1167. - PubMed
-
- Artaza JN, Reisz-Porszasz S, Dow JS, Kloner RA, Tsao J, Bhasin S, Gonzalez-Cadavid NF. Alterations in myostatin expression are associated with changes in cardiac left ventricular mass but not ejection fraction in the mouse. J Endocrinol. 2007;194:63–76. - PubMed
-
- Catalucci D, Latronico MV, Ellingsen O, Condorelli G. Physiological myocardial hypertrophy: how and why? Front Biosci. 2008;13:312–324. - PubMed
-
- Chandra M, Rundell VL, Tardiff JC, Leinwand LA, De Tombe PP, Solaro RJ. Ca2+ activation of myofilaments from transgenic mouse hearts expressing R92Q mutant cardiac troponin T. Am J Physiol Heart Circ Physiol. 2001;280:H705–713. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U24 DK076126/DK/NIDDK NIH HHS/United States
- R01 HL075643/HL/NHLBI NIH HHS/United States
- AR051917/AR/NIAMS NIH HHS/United States
- HL085686/HL/NHLBI NIH HHS/United States
- R01 HL084642/HL/NHLBI NIH HHS/United States
- R01 HL064387/HL/NHLBI NIH HHS/United States
- R01 HL085686/HL/NHLBI NIH HHS/United States
- R03 AR051917/AR/NIAMS NIH HHS/United States
- R24 HL064387/HL/NHLBI NIH HHS/United States
- HL03174/HL/NHLBI NIH HHS/United States
- HL64387/HL/NHLBI NIH HHS/United States
- DK076126/DK/NIDDK NIH HHS/United States
- HL084642/HL/NHLBI NIH HHS/United States
- P01 HL003174/HL/NHLBI NIH HHS/United States
- HL075643/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

