Cu(I)-catalyzed Huisgen azide-alkyne 1,3-dipolar cycloaddition reaction in nucleoside, nucleotide, and oligonucleotide chemistry
- PMID: 19737023
- PMCID: PMC2741614
- DOI: 10.1021/cr9001462
Cu(I)-catalyzed Huisgen azide-alkyne 1,3-dipolar cycloaddition reaction in nucleoside, nucleotide, and oligonucleotide chemistry
Figures


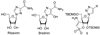


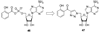
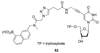
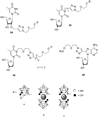

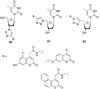
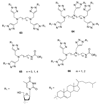
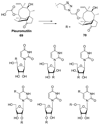
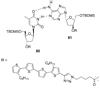





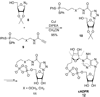
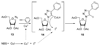

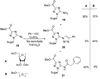

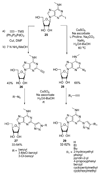



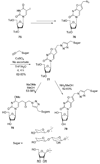
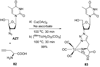
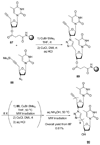
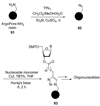

References
-
- Huisgen R, Szeimies G, Möbius L. Chem.Ber. 1967;100:2494.
-
-
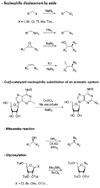
For reviews see: Kolb HC, Sharpless KB. Drug Discovery Today. 2003;8:1128. Bock VD, Hiemstra H, van Maarseveen JH. Eur. J. Org. Chem. 2006;51 Binder WH, Kluger C. Curr. Org. Chem. 2006;10:1791. Binder WH, Sachsenhofer R. Macromol. Rapid Commun. 2007;28:15. Moorhouse AD, Moses JE. Chem. Med. Chem. 2008;3:715. Binder WH, Sachsenhofer R. Macromol. Rapid Commun. 2008;29:952. Lutz J-F, Zarafshani Z. Adv.Drug. Deliv. Rev. 2008;60:958.
-
-
- Kolb HC, Finn MG, Sharpless KB. Angew.Chem. 2001;40:2004. - PubMed
-
-
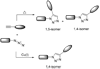
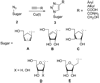
For Pd-catalyzed Sonogashira reaction on nucleosides see: Agrofoglio LA, Gillaizeau I, Saito Y. Chem. Rev. 2003;103:1875. For the preparation of alkynes from aldehydes: Roth GJ, Liepold B, Mömlller SG, Bestmann HJ. Synthesis. 2004;59 For a review on the preparation of azidonucleosides see: Pathak T. Chem. Rev. 2002;102:1623.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

