Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model
- PMID: 19737072
- PMCID: PMC2813150
- DOI: 10.1089/ten.TEA.2009.0518
Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model
Abstract
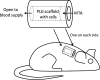
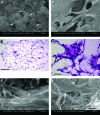
The ultimate goal of this study is to regenerate lost dental pulp and dentin via stem/progenitor cell-based approaches and tissue engineering technologies. In this study, we tested the possibility of regenerating vascularized human dental pulp in emptied root canal space and producing new dentin on existing dentinal walls using a stem/progenitor cell-mediated approach with a human root fragment and an immunocompromised mouse model. Stem/progenitor cells from apical papilla and dental pulp stem cells were isolated, characterized, seeded onto synthetic scaffolds consisting of poly-D,L-lactide/glycolide, inserted into the tooth fragments, and transplanted into mice. Our results showed that the root canal space was filled entirely by a pulp-like tissue with well-established vascularity. In addition, a continuous layer of dentin-like tissue was deposited onto the canal dentinal wall. This dentin-like structure appeared to be produced by a layer of newly formed odontoblast-like cells expressing dentin sialophosphoprotein, bone sialoprotein, alkaline phosphatase, and CD105. The cells in regenerated pulp-like tissue reacted positively to anti-human mitochondria antibodies, indicating their human origin. This study provides the first evidence showing that pulp-like tissue can be regenerated de novo in emptied root canal space by stem cells from apical papilla and dental pulp stem cells that give rise to odontoblast-like cells producing dentin-like tissue on existing dentinal walls.
Figures






References
-
- Mooney D.J. Powell C. Piana J. Rutherford B. Engineering dental pulp-like tissue in vitro. Biotechnol Prog. 1996;12:865. - PubMed
-
- Bohl K.S. Shon J. Rutherford B. Mooney D.J. Role of synthetic extracelluar matrix in development of engineered dental pulp. J Biomaterials Science. (Polymer Edition) 1998;9:749. - PubMed
-
- Buurma B. Gu K. Rutherford R.B. Transplantation of human pulpal and gingival fibroblasts attached to synthetic scaffolds. Eur J Oral Sci. 1999;107:282. - PubMed
-
- Huang G.T.-J. Apexification: the beginning of its end. Int Endod J. 2009;42:855. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

