The formation of cysteine-linked dimers of BST-2/tetherin is important for inhibition of HIV-1 virus release but not for sensitivity to Vpu
- PMID: 19737401
- PMCID: PMC2754425
- DOI: 10.1186/1742-4690-6-80
The formation of cysteine-linked dimers of BST-2/tetherin is important for inhibition of HIV-1 virus release but not for sensitivity to Vpu
Abstract
Background: The Human Immunodeficiency virus type 1 (HIV-1) Vpu protein enhances virus release from infected cells and induces proteasomal degradation of CD4. Recent work identified BST-2/CD317 as a host factor that inhibits HIV-1 virus release in a Vpu sensitive manner. A current working model proposes that BST-2 inhibits virus release by tethering viral particles to the cell surface thereby triggering their subsequent endocytosis.
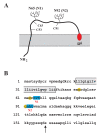
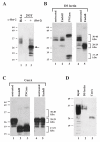
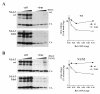
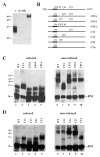
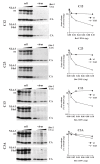
Results: Here we defined structural properties of BST-2 required for inhibition of virus release and for sensitivity to Vpu. We found that BST-2 is modified by N-linked glycosylation at two sites in the extracellular domain. However, N-linked glycosylation was not important for inhibition of HIV-1 virus release nor did it affect surface expression or sensitivity to Vpu. Rodent BST-2 was previously found to form cysteine-linked dimers. Analysis of single, double, or triple cysteine mutants revealed that any one of three cysteine residues present in the BST-2 extracellular domain was sufficient for BST-2 dimerization, for inhibition of virus release, and sensitivity to Vpu. In contrast, BST-2 lacking all three cysteines in its ectodomain was unable to inhibit release of wild type or Vpu-deficient HIV-1 virions. This defect was not caused by a gross defect in BST-2 trafficking as the mutant protein was expressed at the cell surface of transfected 293T cells and was down-modulated by Vpu similar to wild type BST-2.
Conclusion: While BST-2 glycosylation was functionally irrelevant, formation of cysteine-linked dimers appeared to be important for inhibition of virus release. However lack of dimerization did not prevent surface expression or Vpu sensitivity of BST-2, suggesting Vpu sensitivity and inhibition of virus release are separable properties of BST-2.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

