Caspases and kinases in a death grip
- PMID: 19737514
- PMCID: PMC3390419
- DOI: 10.1016/j.cell.2009.08.021
Caspases and kinases in a death grip
Abstract
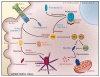
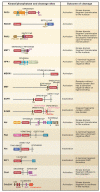
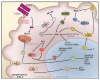
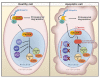
The complex process of apoptosis is orchestrated by caspases, a family of cysteine proteases with unique substrate specificities. Accumulating evidence suggests that cell death pathways are finely tuned by multiple signaling events, including direct phosphorylation of caspases, whereas kinases are often substrates of active caspases. Importantly, caspase-mediated cleavage of kinases can terminate prosurvival signaling or generate proapoptotic peptide fragments that help to execute the death program and facilitate packaging of the dying cells. Here, we review caspases as kinase substrates and kinases as caspase substrates and discuss how the balance between cell survival and cell death can be shifted through crosstalk between these two enzyme families.
Figures
References
-
- Allan LA, Clarke PR. Phosphorylation of caspase-9 by CDK1/cyclin B1 protects mitotic cells against apoptosis. Mol. Cell. 2007;26:301–310. - PubMed
-
- Allan LA, Morrice N, Brady S, Magee G, Pathak S, Clarke PR. Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK MAPK. Nat. Cell Biol. 2003;5:647–654. - PubMed
-
- Arnold R, Liou J, Drexler HC, Weiss A, Kiefer F. Caspase-mediated cleavage of hematopoietic progenitor kinase 1 (HPK1) converts an activator of NFκB into an inhibitor of NFκB. J. Biol. Chem. 2001;276:14675–14684. - PubMed
-
- Arnold R, Frey CR, Müller W, Brenner D, Krammer PH, Kiefer F. Sustained JNK signaling by proteolytically processed HPK1 mediates IL-3 independent survival during monocytic differentiation. Cell Death Differ. 2007;14:568–575. - PubMed
-
- Alvarado-Kristensson M, Andersson T. Protein phosphatase 2A regulates apoptosis in neutrophils by dephosphorylating both p38 MAPK and its substrate caspase 3. J. Biol. Chem. 2005;280:6238–6244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

