The effects of chronic methylphenidate administration on operant test battery performance in juvenile rhesus monkeys
- PMID: 19737611
- PMCID: PMC2942084
- DOI: 10.1016/j.ntt.2009.08.011
The effects of chronic methylphenidate administration on operant test battery performance in juvenile rhesus monkeys
Abstract
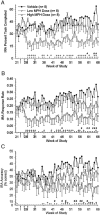
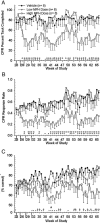
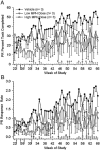
Methylphenidate (MPH) is an amphetamine derivative widely prescribed for the treatment of attention deficit-hyperactivity disorder. Recent concern over its genotoxic potential in children [11] spurred a study on the effects of chronic MPH treatment in a nonhuman primate population and the studies reported here were conducted in conjunction with that study in the same animals. Here, the focus was on the ability of juvenile rhesus monkeys to learn how to perform a battery of operant behavioral tasks while being treated chronically with MPH. Performance of the National Center for Toxicological Research (NCTR) Operant Test Battery (OTB) was used to quantify the learning of tasks thought to model specific aspects of cognitive function including learning, motivation, color and position discrimination, and short-term memory. The OTB tasks designed to assess these specific behaviors included Incremental Repeated Acquisition (IRA), Progressive Ratio (PR), Conditioned Position Responding (CPR), and Delayed Matching-to-Sample (DMTS), respectively. Juvenile males (n=10/group) pressed levers and press-plates for banana-flavored food pellets. Subjects were treated orally, twice a day, five days per week (M-F) for 66 weeks with escalating doses (0.15 mg/kg initially, increased to 2.5 mg/kg for the low dose group and to 12.5 mg/kg for the high dose group) and tested in OTB tasks 30 to 60 min after the morning dose. The findings indicate that MPH at doses up to 2.5 mg/kg twice per day were well tolerated (performance was no different than controls) but at doses of 12.5 mg/kg twice per day there was a significant decrement in OTB performance, characterized by decreases in both percent task completed and response rates for all tasks. These effects of MPH seem primarily due to decreases in motivation to perform for food, which is not surprising given the well known appetite suppressing effects of amphetamine-like stimulants. Thus, the current data do not strongly suggest cognitive impairments following chronic MPH administration.
Copyright (c) 2009 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys.Neurotoxicol Teratol. 2011 Mar-Apr;33(2):220-30. doi: 10.1016/j.ntt.2011.01.001. Epub 2011 Jan 15. Neurotoxicol Teratol. 2011. PMID: 21241795 Free PMC article.
-
Use of the NCTR Operant Test Battery in nonhuman primates.Neurotoxicol Teratol. 1990 Sep-Oct;12(5):413-8. doi: 10.1016/0892-0362(90)90002-t. Neurotoxicol Teratol. 1990. PMID: 2247027
-
Effects of morphine sulfate on operant behavior in rhesus monkeys.Pharmacol Biochem Behav. 1991 Jan;38(1):77-83. doi: 10.1016/0091-3057(91)90592-p. Pharmacol Biochem Behav. 1991. PMID: 2017457
-
Acute behavioral effects of MK-801 in rhesus monkeys: assessment using an operant test battery.Pharmacol Biochem Behav. 1994 Aug;48(4):935-40. doi: 10.1016/0091-3057(94)90203-8. Pharmacol Biochem Behav. 1994. PMID: 7972299
-
Acute effects of caffeine on several operant behaviors in rhesus monkeys.Pharmacol Biochem Behav. 1993 Nov;46(3):733-7. doi: 10.1016/0091-3057(93)90570-j. Pharmacol Biochem Behav. 1993. PMID: 8278453
Cited by
-
Methylphenidate alleviates manganese-induced impulsivity but not distractibility.Neurotoxicol Teratol. 2017 May;61:17-28. doi: 10.1016/j.ntt.2017.03.005. Epub 2017 Mar 28. Neurotoxicol Teratol. 2017. PMID: 28363668 Free PMC article.
-
Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys.Neurotoxicol Teratol. 2011 Mar-Apr;33(2):220-30. doi: 10.1016/j.ntt.2011.01.001. Epub 2011 Jan 15. Neurotoxicol Teratol. 2011. PMID: 21241795 Free PMC article.
-
Dissociable rate-dependent effects of oral methylphenidate on impulsivity and D2/3 receptor availability in the striatum.J Neurosci. 2015 Mar 4;35(9):3747-55. doi: 10.1523/JNEUROSCI.3890-14.2015. J Neurosci. 2015. PMID: 25740505 Free PMC article.
-
Regulation of emotional response in juvenile monkeys treated with fluoxetine: MAOA interactions.Eur Neuropsychopharmacol. 2016 Dec;26(12):1920-1929. doi: 10.1016/j.euroneuro.2016.10.010. Epub 2016 Nov 13. Eur Neuropsychopharmacol. 2016. PMID: 27852517 Free PMC article.
-
Development of a physiologically based model to describe the pharmacokinetics of methylphenidate in juvenile and adult humans and nonhuman primates.PLoS One. 2014 Sep 3;9(9):e106101. doi: 10.1371/journal.pone.0106101. eCollection 2014. PLoS One. 2014. PMID: 25184666 Free PMC article.
References
-
- Arnsten AF. Stimulants: therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31(11):2376–2383. - PubMed
-
- Arnsten AF. Fundamentals of attention-deficit/hyperactivity disorder: circuits and pathways. J. Clin. Psychiatry. 2006;67(Suppl 8):7–12. - PubMed
-
- Barkley RA, McMurray MB, Edelbrock CS, Robbins K. Side effects of methylphenidate in children with attention deficit hyperactivity disorder: a systemic, placebo-controlled evaluation. Pediatrics. 1990;86:184–192. - PubMed
-
- Bedford JA, Marquis DK, Wilson MC. The effects of several anorexigenics on monkey social behavior. Pharmacol. Biochem. Behav. 1984;20(3):317–321. - PubMed
-
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol. Psychiatry. 2006;60(10):1111–1120. 15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

