Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis
- PMID: 19737863
- PMCID: PMC2757872
- DOI: 10.1084/jem.20090545
Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis
Abstract
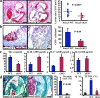
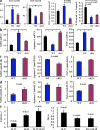
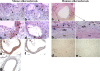
Atherosclerosis is an inflammatory vascular disease responsible for the first cause of mortality worldwide. Recent studies have clearly highlighted the critical role of the immunoinflammatory balance in the modulation of disease development and progression. However, the immunoregulatory pathways that control atherosclerosis remain largely unknown. We show that loss of suppressor of cytokine signaling (SOCS) 3 in T cells increases both interleukin (IL)-17 and IL-10 production, induces an antiinflammatory macrophage phenotype, and leads to unexpected IL-17-dependent reduction in lesion development and vascular inflammation. In vivo administration of IL-17 reduces endothelial vascular cell adhesion molecule-1 expression and vascular T cell infiltration, and significantly limits atherosclerotic lesion development. In contrast, overexpression of SOCS3 in T cells reduces IL-17 and accelerates atherosclerosis. We also show that in human lesions, increased levels of signal transducer and activator of transcription (STAT) 3 phosphorylation and IL-17 are associated with a stable plaque phenotype. These results identify novel SOCS3-controlled IL-17 regulatory pathways in atherosclerosis and may have important implications for the understanding of the increased susceptibility to vascular inflammation in patients with dominant-negative STAT3 mutations and defective Th17 cell differentiation.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

