Bacillus anthracis capsule activates caspase-1 and induces interleukin-1beta release from differentiated THP-1 and human monocyte-derived dendritic cells
- PMID: 19737897
- PMCID: PMC2798191
- DOI: 10.1128/IAI.00956-09
Bacillus anthracis capsule activates caspase-1 and induces interleukin-1beta release from differentiated THP-1 and human monocyte-derived dendritic cells
Abstract
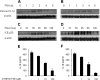
The poly-gamma-d-glutamic acid (PGA) capsule is one of the major virulence factors of Bacillus anthracis, which causes a highly lethal infection. The antiphagocytic PGA capsule disguises the bacilli from immune surveillance and allows unimpeded growth of bacilli in the host. Recently, efforts have been made to include PGA as a component of anthrax vaccine; however, the innate immune response of PGA itself has been poorly investigated. In this study, we characterized the innate immune response elicited by PGA in the human monocytic cell line THP-1, which was differentiated into macrophages with phorbol 12-myristate 13-acetate (PMA) and human monocyte-derived dendritic cells (hMoDCs). PGA capsules were isolated from the culture supernatant of either the pXO1-cured strain of B. anthracis H9401 or B. licheniformis ATCC 9945a. PGA treatment of differentiated THP-1 cells and hMoDCs led to the specific extracellular release of interleukin-1beta (IL-1beta) in a dose-dependent manner. Evaluation of IL-1beta processing by Western blotting revealed that cleaved IL-1beta increased in THP-1 cells and hMoDCs after PGA treatment. Enhanced processing of IL-1beta directly correlated with increased activation of its upstream regulator, caspase-1, also known as IL-1beta-converting enzyme (ICE). The extracellular release of IL-1beta in response to PGA was ICE dependent, since the administration of an ICE inhibitor prior to PGA treatment blocked induction of IL-1beta. These results demonstrate that B. anthracis PGA elicits IL-1beta production through activation of ICE in PMA-differentiated THP-1 cells and hMoDCs, suggesting the potential for PGA as a therapeutic target for anthrax.
Figures
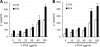
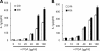
References
-
- Brittingham, K. C., G. Ruthel, R. G. Panchal, C. L. Fuller, W. J. Ribot, T. A. Hoover, H. A. Young, A. O. Anderson, and S. Bavari. 2005. Dendritic cells endocytose Bacillus anthracis spores: implications for anthrax pathogenesis. J. Immunol. 174:5545-5552. - PubMed
-
- Cerretti, D. P., C. J. Kozlosky, B. Mosley, N. Nelson, K. Van Ness, T. A. Greenstreet, C. J. March, S. R. Kronheim, T. Druck, L. A. Cannizzaro, K. Huebner, and R. A. Black. 1992. Molecular cloning of the interleukin-1β-converting enzyme. Science 256:97-100. - PubMed
-
- Chabot, D. J., A. Scorpio, S. A. Tobery, S. F. Little, S. L. Norris, and A. M. Friedlander. 2004. Anthrax capsule vaccine protects against experimental infection. Vaccine 23:43-47. - PubMed
-
- Chakrabarty, K., W. Wu, J. L. Booth, E. S. Duggan, K. M. Coggeshall, and J. P. Metcalf. 2006. Bacillus anthracis spores stimulate cytokine and chemokine innate immune responses in human alveolar macrophages through multiple mitogen-activated protein kinase pathways. Infect. Immun. 74:4430-4438. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

